Abstract
Objectives: Long term acute care hospitals (LTACHs) cater to chronically, critically ill patients with prolonged durations of stay, significant co-morbidities and high rates of intravenous antibiotic utilization. Transmission dynamics between LTACHs and acute care hospitals (ACHs) have shown significant inter-relatedness. Consequently, LTACHs may act as reservoirs for multi-drug resistant organisms (MDR) organisms including P. aeruginosa and Enterobacteriaceae compared to ACHs. Choosing appropriate empirical therapy for these MDROs can lower mortality but is unpredictable and problematic. Recently, two new antimicrobials, ceftolozane-tazobactam (C/T) and ceftazidime-avibactam (CZA), both with in vitro activity against Enterobacteriaceae and P. aeruginosa , have been approved. To ascertain “real world” information about these organisms in LTACHs, we tested C/T and CZA against carbapenem-resistant Enterobacteriaceae and ceftazidime non-susceptible P. aeruginosa obtained from clinical isolates of infected patients at 12 LTACH’s.
Design: Strains were isolated from unique clinical specimens submitted by twelve regional LTACHs to a centralized microbiology laboratory in southern California between March and December 2015. ETests were performed according to the manufacturers’ instructions and interpreted according to FDA criteria.
Results: CZA had very good activity against ESBL and CRE Enterobacteriaceae . For carbapenem-resistant Klebsiella pneumoniae,the MIC90 for CZA was 4/4 mcg/mL. CZA was active in vitro against carbapenem-resistant Enterobacteriaceae and to a lesser extent against ceftazidime resistant P.aeruginosa.C/T (88% susceptible) appeared to demonstrate better in vitro activity against ceftazidime non-susceptible strains of P. aeruginosa than did CZA (72% susceptible). There were no isolates that were susceptible to CZA but resistant to C/T.
Key words
ceftolozane tazobactam, p.aeruginosa,MDRO, LTACHs(Long term Acute Care Hospital), resistance, ceftazidime avibactam, pseudomonas resistance,CRE
Introduction
Multi-Drug Resistant (MDR) Gram-negative aerobic bacteria have become a worldwide concern that has been identified as an urgent global threat by the Centers for Disease Control and Prevention (CDC) [1]. This includes Enterobacteriaceae with β-lactam resistance due to extended-spectrum β-lactamases (ESBLs), carbapenem resistance (KPC, NDM, AmpC + porin changes) as well as MDR P. aeruginosa, including strains resistant to ceftazidime (AmpC and some OprD lacking isolates). The Infectious Disease Society of America (IDSA) launched two campaigns, “Bad Drugs-No Bugs” and “10 × 20” to encourage the development of new agents to combat this threat [2]. In the past two years, two new antimicrobials, ceftolozane-tazobactam (C/T) and ceftazidime-avibactam (CZA), both with in vitro activity against Enterobacteriaceae and P. aeruginosa, have been approved for complicated intra-abdominal infections (cIAI) and complicated urinary tract infections (cUTI) with Phase 3 nosocomial pneumonia trials ongoing [3]. Ceftolozane is a novel β-lactam that was combined with tazobactam, an established penicillanic acid sulfone β-lactamase inhibitor. Ceftazidime is an established cephalosporin combined with avibactam, a new non- β-lactam β-lactamase inhibitor that inactivates select β-lactamases and protects ceftazidime from degradation. Concerns about the development of resistance to these newer agents have been raised with there being limited real-world clinical data available on their in vitro activity. This limited information is, in part, due to the lack of FDA approved commercially available tests for these newer antimicrobial agents [4].
Long term acute care hospitals (LTACHs) cater to chronically, critically ill patients who may have prolonged durations of stay, significant co-morbidities and high rates of intravenous antibiotic utilization. Approximately 50% of short term acute care (STACHs) patients with CRE were ultimately discharged to LTACHs so that, not surprisingly, there is a “9-fold higher prevalence rate of colonization” at LTACHS compared to STACHs [5]. Within Southern California LTACHs there is a 39.8% respiratory failure rate, 64.8% have tracheostomies and 50.9% have a central line in place [Han et al 2017] [5]. Consequently, LTACHs have an increased potential for serving as reservoirs for infections with MDR organisms, and the heavy exposure to antimicrobials and longer lengths of stay are likely to lead to increased prevalence and development of unique resistance patterns [5,6]. Han et al. [5] recently noted a higher incidence of resistant organisms for LTACHs compared to STACHs. Because of this greater propensity for antimicrobial resistance, we therefore studied the in vitro activity of C/T and CZA against clinical isolates of ceftazidime non-susceptible P. aeruginosa (tested C/T and CZA) and ESBL or carbapenem-resistant (CRE) enterobacteriaceae (tested CZA only) isolates obtained from LTACH patients.
Methods
Test strains, enterobacteriaceae isolates that were ESBL or CRE and P. aeruginosa isolates non-susceptible to ceftazidime, were isolated from clinical specimens submitted by twelve regional LTACHs to a centralized microbiology laboratory in Southern California between March and December 2015. Specimens were processed, and isolates were identified by standard criteria [7]. Duplicate isolates from the same patient were excluded. All isolates were identified by the VITEK® 2 with the GN card and initial susceptibility testing was performed with the AST-GN67 card according to manufacturer’s instructions (bioMerieux, Durham, NC) and interpretations based on established guidelines [8]. C/T and CZA ETESTS® were obtained from Merck & Co, Inc. and Allergan, respectively, through IHMA (labeled for Research Use Only) and susceptibility tests were performed according to the manufacturer’s recommendations were tested. Briefly: (a) A 0.5 McFarland suspension was prepared in sterile saline; (b) The inoculum was spread over the entire surface of a Mueller-Hinton II agar plate; (c) ETEST strips were placed onto the surface; (d) Plates were incubated in non-CO2 at 37°C for 16-24 hours; (e) The MIC was read as the value where the ellipse of inhibition met the ETEST strip. Quality control tests using escherichia coli ATCC 25922, K. pneumoniae ATCC 700603 and P. aeruginosa ATCC 27853 were performed daily with acceptable results according to manufacturer’s recommendations. C/T interpretive criteria for P. aeruginosa is ≤ 4/4 mcg/mL susceptible, 8/4 mcg/mL intermediate, and ≥ 16/4 mcg/mL resistant. CZA interpretive criteria for both Enterobacteriaceae and P. aeruginosa are ≤ 8/4 mcg/mL susceptible and ≥ 16/4 mcg/mL resistant.
Only the activity of CZA was tested against the enterobacteriaceae isolates, whereas both the activity of CZA and C/T were tested in P. aeruginosa isolates. For P. aeruginosa other resistance may be present, but isolates were further characterized only for imipenem resistance.
Results
P. aeruginosa
From the 111 ceftazidime non-susceptible P. aeruginosa isolates, there were 41 ceftazidime intermediate strains (16 mcg/mL) and 70 ceftazidime resistant strains (≥32 mcg/mL). All 111 P. aeruginosa were also non-susceptible to piperacillin/tazobactam (MIC > 16 mcg/ml). Of the ceftazidime resistant strains, 67/70 (96%) and 59/70 (84%) were also resistant to cefepime and imipenem, respectively; 98 of 111 isolates (88%) were susceptible to C/T. For isolates that were ceftazidime intermediate, 37/41 (90%) were susceptible to C/T and 34/41 (83%) were susceptible to CZA. For ceftazidime resistant isolates, 61/70 (87%) were susceptible to C/T and 46/70 (66%) were susceptible to CZA (Figure 1). There were no isolates that were susceptible to CZA but resistant to C/T. There were 107 isolates with imipenem susceptibility data; of these, 28/41 (68%) of the ceftazidime intermediate isolates were resistant to imipenem; of the 66 ceftazidime resistant isolates, 54 (82%) were imipenem resistant. Of the imipenem resistant isolates, 71/82 (87%) were susceptible to C/T while only 56/82 (68%) were susceptible to CZA. Overall, C/T (88% susceptible) appeared to demonstrate better in vitro activity against ceftazidime non-susceptible strains of P. aeruginosa than did CZA (72% susceptible). No P. aeruginosa isolates were subject to molecular testing to determine resistance mechanisms.
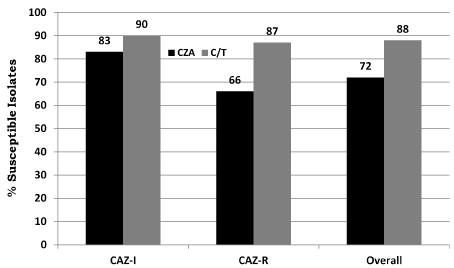
Figure 1. Comparison of percent susceptible to C/T and CZA vs ceftazidime (CAZ) non-susceptible P. aeruginosa isolates from 12 California LTACHs.
LTACH: Long Term Acute Care Hospitals, C/T: Ceftolozane Tazobactam, CZA: Ceftazidime-Avibactam, CAZ-I: intermediate (MIC < 16 mcg/mL), R: Resistant (MIC >32 mcg/ mL) FDA-approved susceptibility breakpoints of ≤4 mcg/mL and ≤8 mcg/mL were used for ceftolozane-tazobactam and ceftazidime-avibactam, respectively.
Enterobacteriaceae
All 29 ESBL-producing enterobacteriaceae (carbapenem-susceptible) strains were susceptible to CZA. There were 245 enterobacteriaceae tested with 242 (98.8%) that were susceptible to CZA (Table 1). Of the 232 carbapenem-resistant K. pneumoniae isolates , 99.6% were CZA susceptible. There was a total of 3 resistant isolates ( K. pneumoniae , 48 mcg/mL; Citrobacter freundii , 32 mcg/mL; E. coli , 16 mcg/mL), but unfortunately all three were lost for subsequent testing and unavailable to confirm initial resistance or to be available for further evaluation of molecular resistance mechanisms. The CZA MIC50 and MIC90 for the carbapenem-resistant K. pneumoniae, respectively, were 2/4 and 4/4 mcg/mL. Overall, CZA had very good activity against ESBL and CRE. In our population of carbapenem-resistant K. pneumoniae , the MIC90 for CZA was 4/4 mcg/mL.
Table 1. The in vitro activity of CZA according to CRE or ESBL resistance, against Enterobacteriaceae strains isolated from 12 California LTACHs.
Enterobacteriaceae |
Resistance Type |
Number of strains Tested |
Percent of Susceptible strains |
Number of Resistant strains |
K. pneumoniae |
CRE |
232 |
99.6 |
1 |
E. coli |
CRE |
5 |
80 |
1 |
C. freundii |
CRE |
3 |
67 |
1 |
E. cloacae |
CRE |
3 |
100 |
0 |
E. aerogenes |
CRE |
1 |
100 |
0 |
R. planticola |
CRE |
1 |
100 |
0 |
K. pneumoniae |
ESBL |
13 |
100 |
0 |
E. coli |
ESBL |
13 |
100 |
0 |
P. mirabilis |
ESBL |
2 |
100 |
0 |
M. morganii |
Other |
1 |
100 |
0 |
CRE: Carbapenem Resistant Enterobacteriaceae, ESBL: Extended Spectrum Beta-Lactamase, LTACH: Long Term Acute Care Hospitals
Discussion
P. aeruginosa has multiple resistance mechanisms, conferring limited therapeutic options choices. The National Healthcare Safety Network (NHSN) estimated that 19.3% of P. aeruginosa isolates in their surveillance studies are intermediate or resistant to carbapenems [9]. While this issue is emerging for STACs, the problem is even more dramatic for LTACHs that receive patients who have been heavily pretreated and exposed to multiple antimicrobial agents and often arrive with already developed resistant pathogen infections [5]. Two new agents active against resistant Gram-negative rods have recently been developed: C/T and CZA. Buehrle et al. [10] recently reported on the activity of both agents against 38 meropenem resistant P. aeruginosa isolates, none of which had carbapenemases and found C/T to be more active than CZA. They found that for P. aeruginosa strains resistant to carbapenems and all β-lactams that C/T was more active than CZA (67% vs 33% susceptible, respectively) [10]. Our study also indicated that C/T appeared more active than CAZ for resistant P. aeruginosa isolated from LTACHs. One study testing MDR P. aeruginosa indicated susceptibility to C/T at 86% while a second study indicated susceptibility of only 68%; [11,12] however, the latter study was comprised of isolates with higher resistance patterns than the US (Israel and parts of Europe). In one CZA report, 67.4% of P. aeruginosa isolates were susceptible if the strains were non-susceptible to ceftazidime, cefepime, piperacillin/tazobactam, and meropenem [13]. In a second study, 97% were susceptible to CZA, but most were ceftazidime susceptible (84%) isolates [14], however, if the isolates were ceftazidime, meropenem, and piperacillin/tazobactam resistant, then the CZA percent susceptible was 72%, but among all MDR P. aeruginosa strains, the CZA percent susceptible was 81%. In a more recent study [Humphries et al. 2017] [15], P. aeruginosa resistant to ceftazidime were shown to be 62% susceptible to C/T while 46% were susceptible to CZA and those strains with resistance to 5 anti-pseudomonal antimicrobials were 57% susceptible to C/T and 28% susceptible to CZA.
Nichols et al. [16] conducted a global surveillance study of P. aeruginosa isolates collected from 2010-2014 and found 8% (563 of 7,062 isolates), including 24.6% of meropenem-non-susceptible isolates, to be resistant to CZA. Miller et al. [17] performed a post-hoc analysis of a randomized phase III double blind trial of C/T plus metronidazole versus meropenem in cIAIs and reported a 100% cure rate (26/26) for C/T treated patient in whom P. aeruginosa was isolated.
More recently, Livermore et al. [2017] [18] assessed the activity of C/T against 6080 British Society for Antimicrobial Chemotherapy (BASC) surveillance isolates and 5471 referred problem organisms. They found C/T was active against 99.8% of the P. aeruginosa surveillance isolates and 99.7% of the referred isolates. They noted that C/T MICs were 2- to 8-fold lower than those of ceftazidime, except for isolates with metallo-beta-lactamases (MBLs) or ESBL. For P. aeruginosa isolates that were resistant to all beta-lactams studied, including ceftazidime and carbapenems, 51.9% were susceptible to C/T and 80.9% if they lacked “ESBL, MB and GES enzymes.”
In the US, Shortridge et al. [19] studied 3,851 P. aeruginosa isolates from 32 US STACs and found 97% of the isolates were C/T susceptible. For meropenem non-susceptible isolates 87.6% were susceptible to C/T compared to 68% of those resistant to all beta-lactams. In comparison we found C/T to be active against 88% of LTACH P. aeruginosa isolates. This suggests the need for continued surveillance of P. aeruginosa LTACH isolates.
Our data showed that for enterobacteriaceae , CZA had 100% susceptibility to ESBL stains and 99.6% susceptibility to CRE strains. Two recent reports support this data. Livermore et al. [20] reported high CZA susceptibility rates to ESBL and CRE isolates with 87% susceptibility to all ceftazidime-resistant enterobacteriaceae . Sader et al. [21] reported 97.5% CZA susceptibility for CRE and > 99% susceptibility for ESBL producing enterobacteriaceae .
Conclusion
These two new agents seem to have different potential uses in LTACHs. CZA appears to be an active agent in vitro against carbapenem-resistant enterobacteriaceae but lesser activity against ceftazidime resistant P . aeruginosa . C/T appears to have better in vitro activity against ceftazidime non-susceptible P. aeruginosa . Continued surveillance and larger studies of LTACH isolates for the emergence of resistance seem warranted.
Transparency declarations and acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or nor-for-profit sectors. We would like to thank the manufacturers for supplying ETest strips (Research Use Only) for this study. We acknowledge the contributions of Ravina Kullar, PharmD, MPH for help in obtaining references and review of this manuscript.
References
- Antibiotic resistance threats in the United States 2014. Centers for Disease Control and Prevention. [http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf]. Accessed: 10-30-17
- Boucher HW, Talbot GH, Benjamin DK, Bradley J,Guidos RJ, et al. (2013) 10 x '20 Progress--development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis 56: 1685-1694. [Crossref]
- Liscio JL, Mahoney MV, Hirsch EB (2015) Ceftolozane/tazobactam and ceftazidime/avibactam: two novel beta-lactam/beta-lactamase inhibitor combination agents for the treatment of resistant Gram-negative bacterial infections. Int J Antimicrob Agents 46: 266-271. [Crossref]
- Humphries RM, Hindl2021 Copyright OAT. All rights reserv antimicrobial agents?…?but No tests! the challenge of antimicrobial susceptibility testing in the current US regulatory landscape. Clin Infect Dis 63: 83-88. [Crossref]
- Han JH, Goldstein EJ, Wise J, Bilker WB, Tolomeo P, et al. (2017) Epidemiology of carbapenem-resistant Klebsiella pneumoniae in a network of Long-Term Acute Care Hospitals. Clin Infect Dis 64: 839-844. [Crossref]
- Rogers MA, Mody L, Chenoweth C, Kaufman SR, Saint S (2008) Incidence of antibiotic-resistant infection in long-term residents of skilled nursing facilities. Am J Infect Control 36: 472-425. [Crossref]
- Jorgensen JH, Pfaller, MA, Carroll KC & American Society for Microbiology (2015) Manual of Clinical Microbiology. (11th edn), Washington, DC: ASM Press.
- Clinical and Laboratory Standards Institute. (2016) Performance standards for antimicrobial susceptibility testing. (26th edn), CLSI document M100. Wayne, PA: CLSI.
- Centers for Disease Control and Prevention (2017) Antibiotic Resistance Patient Safety Atlas - Data on Antibiotic-Resistant Healthcare-Associated Infections. [http://gis.cdc.gov/grasp/PSA/index.html].
- Buehrle DJ, Shields RK, Chen L, Hao B, Press EG, et al. (2016) Evaluation of the In Vitro Activity of Ceftazidime-Avibactam and Ceftolozane-Tazobactam against Meropenem-Resistant Pseudomonas aeruginosa Isolates. Antimicrob Agents Chemother 60: 3227-3231. [Crossref]
- Sutherland C, Nicolau D (2014) In vitro potency of ceftolozane/ tazobactam against Pseudomonas aeruginosa displaying multidrug resistance. Annual Meeting of the Infectious Diseases Society America- ID Week. Philadelphia, PA. Abstract 415. Infectious Diseases Society America. Washington DC.
- Farrell DJ, Sader HS, Catanheira M, Jones RM (2015) Antimicrobial activity of ceftolozane/tazobactam and comparator agents tested against Pseudomonas aeruginosa isolates from 15 European countries and Israel. 25th European Congress of Clinical Microbiology and Infectious Diseases. Copenhagen, DK. Abstract P-1299: 146. European Society of Clinical Microbiology and Infectious Diseases, Basel SW.
- Sader HS, Castanheira M, Farrell DJ, Flamm RK, Jones RN (2015) Ceftazidime-avibactam activity when tested against ceftazidime-nonsusceptible Citrobacter spp., Enterobacter spp., Serratia marcescens, and Pseudomonas aeruginosa from United States medical centers (2011-2014). Diagn Microbiol Infect Dis 83: 389-394. [Crossref]
- Sader HS, Castanheira M, Mendes RE, Flamm RK, Farrell DJ, et al. (2015) Ceftazidime-avibactam activity against multidrug-resistant Pseudomonas aeruginosa isolates in U. S. medical centers in 2012 and 2013. Antimicrob Agents Chemother 5: 3656-3659. [Crossref]
- Humphries RM, Hindler JA, Wong-Beringer A, Miller SA (2017) Activity of Ceftolozane-Tazobactam and Ceftazicime-Avibactam against Beta-Lactam-Resistant Pseudomonas aeruginosa isolates. Antimicrob Agents Chemother 61: e01858-17. [Crossref]
- Nichols WW, de Jonge BL, Kazmierczak KM, Karlowsky JA, Sahm DF (2016) In vitro susceptibility of global surveillance isolates of Pseudomonas aeruginosa to ceftazidime-avibactam (INFORM 2012 to 2014). Antimicrob Agents Chemother 60: 4743-4749. [Crossref]
- Miller B, Popejoy MW, Hershberger E, Steenbergen JN, Alverdy J (2016) Characteristics and outcomes of complicated intra-abdominal infections involving Pseudomonas aeruginosa from a randomized, double-blind, Phase 3 ceftolozane-tazobactam study. Antimicrob Agents Chemother 60: 4387-4390. [Crossref]
- Livermore DM, Mushtaq S, Meunier D, Hopkins KL, Hill R, et al. (2017) Activity of ceftolozane/tazobactam against surveillance and 'problem' Enterobacteriaceae, Pseudomonas aeruginosa and non-fermenters from the British Isles. J Antimicrob Chemother 72: 2278-2289. [Crossref]
- Shortridge D, Castanheira M, Pfaller MA, Flamm RK (2017) Ceftolozane-Tazobactam activity against Pseudomonas aeruginosa clinical isolates from U.S. hospitals: Report from the PACTS Antimicrobial Surveillance Program, 2012 to 2015. Antimicrob Agents Chemother. 61: pii: e00465-17. [Crossref]
- Livermore DM, Meunier D, Hopkins KL, Doumith M, Hill R, et al. (2017) Activity of ceftazidime/avibactam against problem Enterobacteriaceae and Pseudomonas aeruginosa in the UK, 2015-16. J Antimicrob Chemother 73: 648-657. [Crossref]
- Sader HS, Castanheira M, Shortridge D, Mendes RE, Flamm RK (2017) Antimicrobial activity of ceftazidime-avibactam tested against multidrug-resistant Enterobacterieae and Pseudomonas aeruginosa isolates from United States Medical Centers (2013-2016). Antimicrob Agents Chemother 61: pii e01045-17. [Crossref]

