EZH2 is the histone methyltransferase (HMT) that catalyzes the trimethylation of histone H3 lysine 27 (H3K27me3), a histone marker that silences gene expression. Overexpression of EZH2 enhances the growth of malignant cells due to silencing of tumor suppressor genes (TSGs). 3-deazaneplanocin-A (DZNep) blocks the metabolism of methionine resulting in global inhibition of HMTs, including EZH2. This action of DZNep leads to inhibition of growth of malignant cells and reactivation of TSGs. On the other hand, specific inhibitors that target the catalytic site of EZH2: GSK-126, GSK-343, CPI-1205, and tazemetostat (EPZ-6438) were also investigated and exhibited interesting antineoplastic activity. These studies indicated that their anticancer action required a longer duration of treatment than DZNep to exhibit significant antineoplastic activity. This observation suggests that DZNep is a more potent antineoplastic agent than the specific EZH2 inhibitors. Such a difference in anticancer potency may be explained in part by the limited penetration into cells of the specific EZH2 inhibitors due to their large complex molecular structure as compared to the smaller molecular size of DZNep. An additional explanation is that DZNep has several targets in the cell which contribute to its anticancer action: deregulation of methionine metabolism, proteosomal degradation of EZH2, and activation of miRNAs with TSG function. In this study, we compared the in vitro antineoplastic action of DZNep and the specific EZH2 inhibitors using growth inhibition and colony assays on leukemic cells. These assays confirm that DZNep is a more potent anticancer agent than the specific EZH2 inhibitors. DZNep merits clinical investigation in patients with cancer.
epigenetic therapy, EZH2 inhibitors, 3-deazaneplanocin-A, CP-1205, tazemetostat, GSK343, GSK126, antineoplastic action
The polycomb repressive complex 2 (PRC2) is an important regulator of transcription in cells. One of the subunits of PRC2 is enhancer of zeste homolog 2 (EZH2), a histone methyltransferase (HMT) that converts histone H3 lysine 27 to its trimethylated form (H3K27me3), an inhibitor of gene expression. EZH2 can suppress differentiation by repressing lineage-specifying regulators facilitating neoplastic transformation [1]. Dysregulation of EZH2 can lead to the development of malignancy which is due in part to the silencing of tumor suppressor genes (TSGs). Overexpression of EZH2 is observed in different types of cancer and correlates with a poor prognosis [1]. These observations identified EZH2 as an interesting target for chemotherapy and led to the search for inhibitors of this enzyme. The chemical synthesis of 3-deazaneplanocin A (DZNep) identified it as an agent with interesting antiviral activity [2]. DZNep was demonstrated to be a potent inhibitor of S-adenosyl-l-homocysteine (SAH) hydrolase, an enzyme responsible for the conversion of SAH to adenosine and homocysteine [2,3]. This inhibition by DZNep increases the level of SAH in cells, disrupting the metabolism of methionine and reducing the level of S-adenosyl-methionine (SAM). Since SAM is the methyl donor in enzymatic methylation reactions, the end result is a global inhibition of histone methylation, including the reactions catalyzed by EZH2 [4]. Tan et al. [5] were the first to demonstrate that DZNep exhibited very interesting antineoplastic activity against breast carcinoma cells as indicated by induction of apoptosis, reduction in the levels of EZH2 and H3K27me3 and activation of the expression of genes that suppress malignancy. These observations stimulated the research for more specific EZH2 inhibitors that would target its catalytic site. Several specific inhibitors of EZH2 were synthesized and found to exhibits interesting antineoplastic activity in preclinical studies [6-8]. The specific EZH2 inhibitors included: GSK126, GSK343, CP-1205, and tazemetostat (EPZ-6438). Lymphoma cells with increase-in-function mutations in EZH2 were sensitive to the growth inhibitory action of tazemetostat (TAZ) [6]. Several of these catalytic EZH2 inhibitors are under clinical investigation in patients with cancer: GSK126 (NCT020829777); CPI-1205 (NCT03480646); and tazemetostat (NCT03213665). Promising results were obtained in patients with lymphoma treated with tazemetostat and CPI-1205 [9,10]. Are these specific EZH2 inhibitors more potent antineoplastic agents than DZNep? Preclinical studies in animal models with cancer indicated that the specific EZH2 inhibitors required a longer duration of treatment than DZNep to exhibit significant antineoplastic activity [6,7,11-14]. These observations indicated that DZNep perhaps possess more potent anticancer activity. In this report we compared the in vitro antineoplastic activity of DZNep and the specific EZH2 inhibitors on human leukemic cells. Assays on growth inhibition and colony formation indicated that DZNep exhibited more potent antineoplastic activity than any of the specific EZH2 inhibitors. These studies suggest that DZNep also merits clinical investigation in patients with cancer.
Materials and cells
HL-60 human myeloid leukemic cells were maintained in RPMI-1640-HEPES medium (Invitrogen) with 10% FBS. 3-Deazaneplanocin-A (DZNep) was provided by Dr Victor E. Marquez, Chemical Biology Laboratory (Frederick, MD). DZNep was dissolved in 50% sterile phosphate buffer saline (PBS) pH 6.8 (Invitrogen), sterilized by 0.22 micron filtration and stored at -20oC. The specific EZH2 inhibitors GSK126, GSK343, CPI-1205 and tazemetostat (EPZ-6438) were obtained from Xcessbio Biosciences Inc., San Diego, CA; Structural Genomics Corportation (SGC), Toronto; Selleckchem, Houston, TX; and MedKoo Biosciences Inc, Morrisville, NC, respectively. These EZH2 inhibitors were dissolved in dimethyl sulfoxide (DMSO) and stored at -20oC.
Effect of drugs on growth inhibition and colony formation
HL-60 leukemic cells (50,000 or 100,000 cells/ml) were placed in 25 cm2 flasks (Sarstedt) and the drugs added at a concentration of 5 µM. After a 48 h drug exposure a cell count was performed using the Beckman Model Z Coulter Counter. For colony assay, 100 HL-60 cells were placed in 0.36% soft agar medium containing 20% serum in RPMI 1640 medium. The number of colonies (>500 cells) was counted after 18-21 days of incubation. The cloning efficiency was in the range of 60-75%. Due to the limited water solubility of the specific EZH2 inhibitors they were dissolve in DMSO, a solvent that was reported to induce weak induction of differentiation of HL-60 leukemic cells [15]. The final concentration of DMSO in the culture media was 0.1%. Statistical analysis of the data was performed using Prism GraphPad software and Tukey’s multiple comparison test.
Inhibition of growth of HL-60 leukemic cells by DZNep, CPI-1205, tazemetostat, GSK343, or GSK126
The effect of DZNep and the different specific EZH2 inhibitors on the growth of HL-60 myeloid leukemic cells are shown in Figure 1. The leukemic cells were exposed to 5 µM of each of the antineoplastic agents for 48 h. A cell count was performed at 48 h to determine % growth inhibition (n = 3-4). The growth inhibition (mean ± S.E.) for the different agents were: DMSO: 4.8 ± 4.8; DZNep: 60.4 ± 1.7; CPI-1205: 3.6 ± 1.5; tazemetostat: 6.1 ± 3.5; GSK343: 26.5 ± 8.6; GSK126: 12.3 ± 2.7. DMSO was the solvent used to dissolve the specific EZH2 inhibitors produced minor growth inhibition. DZNep was a significantly more potent inhibitor of growth than any of the specific EZH2 inhibitors (p < 0.05). No significant growth inhibition was observed for CPI-1205. GSK343 exhibited more inhibition of growth than GSK126 or tazemetostat.
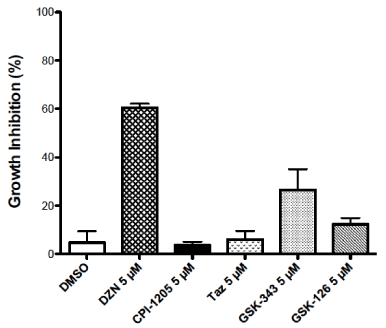
Figure 1. Effect of DZNep (DZN) and specific EZH2 inhibitors: CPI-1205, tazemetostat (TAZ), GSK343 and GSK126 on inhibition of the growth of HL-60 leukemic cells. The inhibitors were added at a concentration of 5 µM. A cell count was made at 48 h using Coulter electronic cell counter. The mean ± S.E. values for each of the inhibitors are indicated (n = 3-4). DZNep was a more potent inhibitor than any of the specific EZH2 inhibitors (p < 0.05)
Inhibition of colony formation of HL-60 leukemic cells by DZNep, CPI-1205, tazemetostat, GSK343, or GSK126
The leukemic cells were exposed to 5 µM of each of the antineoplastic agents for 48 h. At the end of drug exposure, the leukemic cells were placed in soft agar to quantitate colony formation see Figure 2. The mean ± S.E. values for reduction in colony formation (n = 3-4) for these agents were: DMSO: 10.3 ± 1.5; DZNep: 72.3 ± 9.3; CPI-1205: 9.1 ± 3.7; tazemetostat: 17.7 ± 6.0; GSK343: 15.5 ± 7.7; GSK126: 23.2 ± 6.1. DZNep was a significantly more potent inhibitor of colony formation than any of the specific EZH2 inhibitors (p < 0.05). CPI-1205 did not exhibit a significant reduction in colony formation. Analysis of the specific EZH2 inhibitors revealed that GSK126 exhibited more antileukemic activity that GSK343 or tazemetostat.
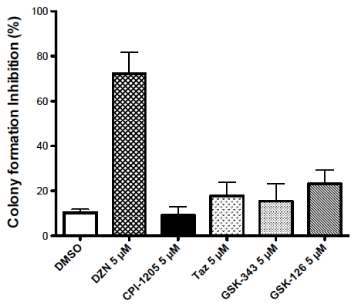
Figure 2. Effect of DZNep (DZN) and specific EZH2 inhibitors: CPI-1205, tazemetostat (TAZ), GSK343 and GSK126 on inhibition of colony formation in soft agar medium of HL-60 leukemic cells. The inhibitors were added at a concentration of 5 µM. A cell count was made at 48 h using Coulter electronic cell counter. An aliquot of 100 cells were placed in soft agar growth medium. A colony count was made on day 18-21. The mean ± S.E. values for each of the inhibitors are indicated (n = 3-4). DZNep was a more potent inhibitor than any of the specific EZH2 inhibitors (p < 0.05)
The results obtained in this report clearly indicate that DZNep exhibits significantly more in vitro antileukemic activity than several inhibitors that target the catalytic site of EZH2: CPI-1205, tazemetostat, GSK343, and GSK126. For the colony assay after the 48 h treatment with the inhibitors, the cells were placed in “drug-free” medium for 18-20 days to permit visible colony formation. The delayed epigenetic action of the inhibitors becomes apparent using this method. Our results which indicate that DZNep is a more potent antineoplastic agent than tazemetostat are in accord with the report that DZNep induced more apoptosis in lymphoma cells than tazemetostat [16].
One aspect of the pharmacology of the specific EZH2 inhibitors that needs an explanation is the long duration of treatment required for these inhibitors to exhibit significant anticancer activity as compared to most standard antineoplastic agents. Due to the very high affinity of these inhibitors for the catalytic site of EZH2 one would expect a moderate to high anticancer potency. Preclinical studies of both tazemetostat and CPI-1205 revealed that a duration of at least 10 days in vitro treatment with these inhibitors was necessary to exhibit significant inhibition of tumor growth [6,7]. Similar results were also observed in a mouse model with xenograft tumors [6,7]. In phase I studies in patients with lymphoma, a 28-day cycle of chemotherapy with tazemetostat or CPI-1205 were used to obtain responses [9,10]. In comparison, a 24 h in vitro treatment of human myeloid leukemic cells by DZNep exhibited significant antineoplastic activity [14]. In the mouse model of leukemia, DZNep administered over a 6 h interval also exhibited significant antineoplastic activity [14].
These results may be explained in part by the differences in the pharmacodynamics between the specific EZH2 inhibitors and DZNep. The specific EZH2 inhibitors are large complex molecules (molecular weight >500) which have poor penetration into cells [17]. This characteristic of the specific EZH2 inhibitors necessitate long drug exposure times to maintain a level high enough in the cell to inhibit EZH2. On the other hand, DZNep is a small molecule (molecular weight <270) with a simplified nucleoside structure that facilitates its penetration into cells.
The molecular mechanism of action of DZNep provides an additional explanation for the greater antineoplastic activity of this nucleoside analogue in comparison to the specific EZH2 inhibitors. DZNep inhibits SAH hydrolase leading to an accumulation of SAH in the cell [3]. SAH acts as a competitive inhibitor of SAM for the catalytic site of HMTs. EZH2 is very sensitive to this inhibition, most likely due to the Ki value of SAH and the Km value of SAM for this target enzyme. Other HMTs may be less sensitive to DZNep, probably due to higher Ki and Km values for SAH and SAM, respectively. However, weak to moderate inhibition of other HMTs may also contribute to antineoplastic action of DZNep. The enzyme kinetic values most likely favor the action of DZNep in a manner that EZH2 is the preferential target. Targeting several HMTs may be one of the reasons why DZNep is a more potent antineoplastic agent than the specific EZH2 inhibitors.
Also contributing to the antineoplastic action of DZNep is its enhancement of proteosomic degradation of EZH2. The complex of SAH-EZH2 may trigger the ubiquitination signal to react with the polycomb repressive complex 2 (PRC2) and its subunit, EZH2, leading to rapid degradation by the proteasome. It should be noted that DZNep induces the expression of PRAJA1, a ubiquitin ligase, that targets EZH2-PRC2 for proteosomal degradation [18]. The action of DZNep on microRNA (miRNA) function can also play a role with respect to its antineoplastic action. Over expression of EZH2 can lead to reduction in the expression of microRNAs (miRNAs) with TSG function. DZNep inhibits this action of EZH2 resulting in an increase in these miRNAs with TSG activity and the inhibition of the growth of different types of malignant cells [19-21].
In summary, DZNep targets several HMT, induces proteosomal degradation of EZH2, and reactivates the expression of miRNAs with TSG function. The multiple targets of DZNep provide a reasonable explanation why DZNep is a more potent antineoplastic agent than the specific EZH2 inhibitors. An additional explanation is the more rapid penetration into cells of DZNep due to its smaller size and simple chemical structure as compared to the specific EZH2 inhibitors. These comments support clinical investigation on DZNep in patients with cancer. Positive responses in cancer patients with DZNep will provide the rationale to improve the effectiveness of epigenetic therapy by using DZNep to enhance the antineoplastic activity of the inhibitor of DNA methylation, decitabine, since preclinical studies on the combination of these two agents exhibit remarkable antineoplastic synergy [14,22,23].
This project was supported by a grant from the Faculté de medicine, Uiniversité de Montréal.
The authors disclose no conflict of interest.
- Kim KH, Roberts CW (2016) Targeting EZH2 in cancer. Nat Med 22: 128-134. [Crossref]
- Tseng CK, Marquez VE, Fuller RW, Goldstein BM, Haines DR, et al. (1989) Synthesis of 3-deazaneplanocin A, a powerful inhibitor of S-adenosylhomocysteine hydrolase with potent and selective in vitro and in vivo antiviral activities. J Med Chem 32: 1442-1446.
- Glazer RI, Hartman KD, Knode MC, Richard MM, Chiang PK, et al. (1986) 3-Deazaneplanocin: a new and potent inhibitor of S-adenosylhomocysteine hydrolase and its effects on human promyelocytic leukemia cell line HL-60. Biochem Biophys Res Commun 135: 688-694.
- Miranda TB, Cortez CC, Yoo CB, Liang G, Abe M, et al. (2009) DZNep is a global histone methylation inhibitor that reactivates developmental genes not silenced by DNA methylation. Mol Cancer Ther 8: 1579-1588. [Crossref]
- Tan J, Yang X, Zhuang L, Jiang, X, Wei Chen W, et al. (2007) Pharmacologic disruption of Polycomb- repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev 21: 1050-1063.
- Knutson SK, Kawano S, Minoshima Y (2014) Selective inhibition of EZH2 by EPZ-6438 leads to potent antitumor activity in EZH2-mutant non-Hodgkin lymphoma. Mol Cancer Ther 13: 842-854.
- Vaswani RG, Gehling VS, Dakin LA, Cook AS, Nasveschuk CG, et al. (2016) Identification of (R)-N-((4-Methoxy-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-2-methyl-1-(1-(1-(2,2,2-trifluoroethyl)piperidin-4-yl)ethyl)-1H-indole-3-carboxamide (CPI-1205), a potent and selective inhibitor of histone methyltransferase EZH2, suitable for phase I Clinical Trials for B-Cell Lymphomas. J Med Chem 59: 9928-9941.
- Gulati N, Béguelin W, Giulino-Roth L (2018) Enhancer of zeste homolog 2 (EZH2) inhibitors. Leuk Lymphoma 59: 1574-1585. [Crossref]
- Italiano A, Soria JC, Toulmonde M, Michot JM, Lucchesi C, et al. (2018) Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol 19: 649-659. [Crossref]
- Harb W, Abramson J, Lunning M, Goy A, Maddocks K, et al. (2018) A phase 1 study of CPI-1205, a small molecule inhibitor of EZH2, preliminary safety in patients with B-cell lymphomas. Annals of Oncology 29: 420.
- Yu T, Wang Y, Hu Q, Wu W, Wu Y, et al. (2017) The EZH2 inhibitor GSK343 suppresses cancer stem-like phenotypes and reverses mesenchymal transition in glioma cells. Oncotarget 8: 98348-98359.
- Kurmasheva RT, Sammons M, Favours E, Wu J, Kurmashev D, et al. (2017) Initial testing (stage 1) of tazemetostat (EPZ-6438), a novel EZH2 inhibitor, by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer 64.
- Zeng D, Liu M, Pan J (2017) Blocking EZH2 methylation transferase activity by GSK126 decreases stem cell-like myeloma cells. Oncotarget 8: 3396-3411. [Crossref]
- Momparler RL, Idaghdour Y, Marquez VE, Momparler LF (2012) Synergistic antileukemic action of inhibitors of DNA methylation and histone methylation. Leuk Res 36: 1049-1054.
- Tarella C, Ferrero D, Gallo E, Pagliardi GL, Ruscetti FW (1982) Induction of differentiation of HL-60 cells by dimethyl sulfoxide: evidence for a stochastic model not linked to the cell division cycle. Cancer Res 42: 445-449.
- Akpa CA, Kleo K, Lenze D, Oker E (2019) DZNep-mediated apoptosis in B-cell lymphoma is independent of the lymphoma type, EZH2 mutation status and MYC, BCL2 or BCL6 translocations. PLoS One 14: e0220681. [Crossref]
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46: 3-26.
- Zoabi M, Sadeh R, de Bie P, Marquez VE, Ciechanover A (2011) PRAJA1 is a ubiquitin ligase for the polycomb repressive complex 2 proteins. Biochem Biophys Res Commun 408: 393-398.
- Cao Q, Mani RS, Ateeq B, Dhanasekaran SM, Asangani IA, et al. (2011) Coordinated regulation of polycomb group complexes through microRNAs in cancer. Cancer Cell 20: 187-199.
- Smits M, Nilsson J, Mir SE, van der Stoop PM, Hulleman E, et al. (2010) miR-101 is down-regulated in glioblastoma resulting in EZH2-induced proliferation, migration, and angiogenesis. Oncotarget 1: 710-720.
- Vella S, Pomella S, Leoncini PP, Colletti M, Conti B, et al. (2015) MicroRNA-101 is repressed by EZH2 and its restoration inhibits tumorigenic features in embryonal rhabdomyosarcoma. Clin Epigenetics 7: 82.
- Fernandes do Nascimento AS, Cote S, Jeong LS, Yu J, Momparler RL (2016) Synergistic antineoplastic action of 5-aza-2'deoxycytidine (decitabine) in combination with different inhibitors of enhancer of zeste homolog 2 (EZH2) on human lung carcinoma cells. J Cancer Res Ther 4: 42-49.
- Momparler RL, Côté S, Momparler LF, Idaghdour Y (2017) Inhibition of DNA and histone methylation by 5-aza-2'-deoxycytidine (decitabine) and 3-deazaneplanocin-A on antineoplastic action and gene expression in myeloid leukemic cells. Frontiers Oncol 7: 19.


