Objective: To evaluate the possibility of extending the liver transplantation criteria in patients with HCC beyond the Milan criteria.
Materials and Methods: Fifty-four cirrhotic patients with HCC were transplanted in our institution using the up-to-seven criteria as the upper limit of transplantability. Results were compared with a similar group of 47 patients who were transplanted using the Milan criteria during the period 2006-2012. HCC recurrence and patient survival rates after liver surgery were analyzed. A comparison between the imaging before liver transplant and explanted liver was also performed.
Results: Three- and five-year survival (0.83 vs. 0.78 ; 0.76 vs 0.63) and HCC recurrence (7.2% vs 8.5%) were similar between the up-to-seven and the Milan criteria groups, respectively. In the up-to-seven group, bigger lesions (p=0.04) and more patients undergoing bridging therapies before liver transplant (p=0.0007) were found. In both groups imaging underestimated disease extension in 9 cases (16,7% vs. 19%) and no significant difference was noted.
Conclusion: Recurrence and survival in patients transplanted with up-to-seven criteria are similar. The up-to-seven criteria enable patients with more advanced HCC to undergo liver transplantation.
liver transplant, HCC, survival, imaging
HCC=hepatocellular carcinoma; UCSF=University of California San Francisco; MR=Magnetic resonance; CT=Computed tomography; RFTA=Radiofrequency thermo-ablation; TACE=Transarterial chemoembolization; SIRT=Selective internal radiation therapy; LT=Liver transplantation; HIFU=High-intensity focused ultrasound; AFP=Alpha-feroprotein; NPV=negative predictive value; RECICL=Response evaluation criteria in cancer of the liver; LI-RADS=Liver imaging reporting and data system; mRECIST=Modified response evaluation criteria in solid tumors; NASH=Non-alcoholic steatohepatitis; MELD=model for end-stage liver disease; HBV=Hepatits B virus; HCV=Hepatitis C virus
Liver transplantation (LT) is the best therapy for the treatment of an early-stage hepatocellular carcinoma (HCC) arising in a liver with intermediate/advanced-stage cirrhotic disease. The need to contextually minimize the risk of post-transplant recurrence and to use a limited number of available organs to transplant has caused the development of stringent criteria for the optimization of the results after transplantation in HCC patients. In 1996, Mazzaferro, et al. introduced the Milan criteria , obtaining an excellent 4-year survival rate of 75% [1]. After the introduction of the Milan criteria, several studies have tried to extend these criteria over the limits of the number and size of HCC lesions without compromising the survival rates. To date, no proposal for expansion, with only the exception of the University of California San Francisco (UCSF) criteria, has reached sufficient numbers to assume statistical relevance or ability to amend accepted clinical practice [2,3]. In 2009, Mazzaferro, et al. proposed the up-to-seven criteria for LT, with the cut-off value of seven being the sum of the size of the largest tumor and the number of tumors [4]. Although the up-to-seven criteria have been analyzed all over the world, they have not been as widely accepted as the Milan criteria.
We aimed to compare two groups of transplant patients diagnosed with HCC in our center over two different time frames in which the Milan criteria (2006-2012) and the up-to-seven criteria (2013-2016) were considered as the upper limit of transplantability. Overall survival, recurrence of disease, and the survival in patients experiencing post-transplant recurrence were investigated. We also test the ability of imaging to correctly assess the stage of tumor disease at the time of surgery.
Study population
This prospective/retrospective study was approved by the Institutional Review Board and followed the principles of the Declaration of Helsinki and subsequent amendments. All patients provided written informed consent.
From January 2013 to December 2016, 250 patients with liver cirrhosis were referred to the Liver Transplant Unit of our institution to be scheduled for transplantation.
Inclusion criteria for scheduling were: age younger than 70, alcohol abstention for more than six months, a low-to-intermediate anesthesiological risk for major surgery, and second-level imaging within three months from transplant. In the case of HCC, exclusion criteria were an extra-hepatic disease, the presence of macrovascular infiltration, and a tumor burden exceeding the up-to-seven criteria at the time of surgery.
Of the 79 cirrhotic patients who underwent a deceased-donor liver transplant, we considered in the present study only those with HCC (up-to-seven group). Patients met the up-to-seven criteria from the beginning (waiting list inscription) or after downstaging (Figure 1).
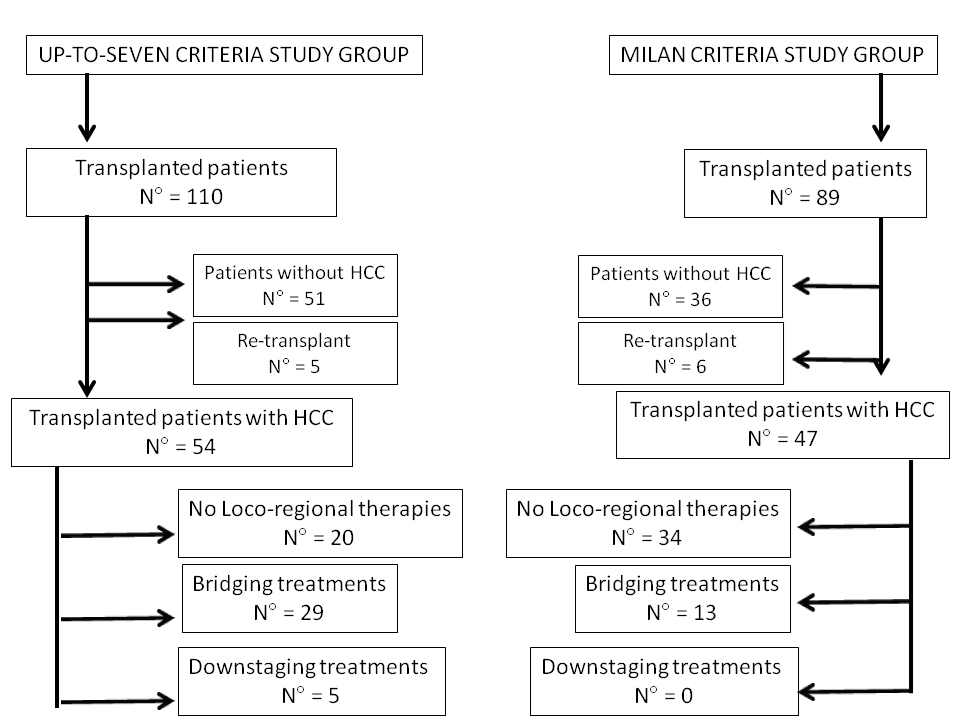
Figure 1. Flow chart of the two study groups
We defined a “bridging” treatment as any locoregional therapy aimed at “stabilizing” the tumor burden within up-to-seven criteria. “Downstaging” treatment was any locoregional therapy aimed at reducing the tumor burden in patients initially out of the up-to-seven criteria, with the intent to decrease their stage.
In all the cases, the tumor burden was evaluated using computed tomography (CT) and/or magnetic resonance (MR). An obligatory imaging control was performed within three months from trans-arterial chemoembolization (TACE) and radiofrequency ablation (RFTA) in downstaged patients. In the case of selective internal radiotherapy (SIRT), patients were required to wait six months before being considered listable for LT. No high-intensity focused ultrasound (HIFU) procedures were reported in the present series. After LT, serum alpha-fetoprotein (AFP) measurement and CT/MR were performed every three months, with the intent to exclude tumor recurrence.
The up-to-seven group was compared with a similar historical group of 47 HCC patients (Milan criteria group) who underwent deceased-donor liver transplantation between January 2006 and December 2012, namely during a period in which the Milan criteria were used as the upper limit for transplantability (Figure 1).
Imaging techniques
CT examination was performed with a multi-detector row scanner (Somatom Sensation 64; Siemens Medical Systems, Erlangen, Germany) using a four-phase protocol [pre-contrast, late arterial phase (30–40 sec.), venous phase (70 sec.) and late dynamic phase (180 sec.)]. A non-ionic high osmolar contrast agent (iomeprol, 400 mg of iodine per milliliter [Iomeron 400; Bracco Imaging, Milan, Italy]) was injected at a dose of 1.3 mL (520 mg of iodine) through an 18-gauge IV catheter inserted into an antecubital vein at 4.5 mL/sec. With the intention to determine the scanning delay for hepatic arterial phase imaging, the bolus transfer time was assessed by using a bolus-tracking technique with automated scan-triggering software (CARE Bolus CT, Siemens MedicalSystems).
MR examination was performed with a 1.5 T magnet (Magnetom Avanto, Siemens Medical Systems) equipped with a 32-channel system, a maximum gradient strength of 45 mT/m, and a peak slew rate of 200 mT/m/ms. The acquisition protocol included T2-weighted turbo spin-echo sequences and T1-weighted gradient-echo sequences with and without fat saturation (both spectral and chemical shift techniques) and T1-weighted 3D gradient-echo post-contrast sequences after liver-specific contrast agent administration (gadobenate dimeglumine 0.1 mL/kg) during the late arterial (30–40 sec.), venous (70 sec.), late dynamic (180 sec.) and hepatobiliary phases (75 min.).
Image analysis
Each dataset of images was evaluated for consensus by three radiologists who were experts in liver imaging (M.D.M. 12-year, C.C. 25-year, P.L. 10-year) according to the liver imaging reporting and data system (LI-RADS) classification for focal liver lesions and the subsequent amendments [5]. Only lesions classified as LI-RADS 5 were considered as HCC and taken into account for staging disease. The response to therapy of treated lesions was assessed according to the modified response evaluation criteria in solid tumors (mRECIST) criteria [6].
Histopathologic Analysis
All explanted livers were analyzed by two experienced pathologists; specimens were sectioned in the axial plane with a slice thickness of 5–10 mm. The preoperative CT and MR findings were directly correlated with pathological findings by an expert radiologist with 20 years of experience in abdominal imaging who was present when the specimens were prepared for evaluation. Microscopic sections were reviewed to confirm the diagnosis of HCC. All lesions were classified according to accepted guidelines [7].
Statistical Analysis
Categorical variables were compared by using the χ² test. Continuous variables were expressed in mean and range and evaluated with the t test. HCC recurrence rates were compared with the χ² test, and three- and five-year survival rates were calculated by using the Kaplan-Meier test and compared with the log-rank test. Survival was defined as the period from the time of operation to the time of death or last censoring. The negative predictive value (NPV) of imaging examination was also calculated. Variables with p<0.05 were considered statistically significant. Statistical analyses were run using the SPSS statistical package version 24.0 (SPSS Inc., Chicago, IL, USA).
Characteristics of the two study groups are summarized in Table 1. Significant differences were found in terms of median largest tumor size (20.3 mm vs. 17.1 mm; p=0.04) and interventional procedures percentage in the up-to-seven group (62 vs. 28%; p=0.0007). In the up-to-seven group more patients with non-alcoholic steatohepatitis (NASH) were transplanted (p=0.03). Gender, age, Child-Pugh score, model for end-stage liver disease (MELD) were similar between the two groups.
Table 1. Characteristics and clinical analysis of the two study groups.
| |
Up-to-seven Criteria |
Milan Criteria |
Transplanted Patients |
110 |
83 |
Transplanted Patients with HCC |
54 49% |
47 56% |
Age |
59 (35-68) |
57 (45-70) |
Sex |
49 Men
5 Women |
43 Men
4 Women |
MELD score |
13 (7-29) [11.6-14.3] |
13.8 (7-22) [12.6-14.9] |
Child-Pugh score: A
B
C |
23 43%
25 46%
6 11 % |
15 32%
24 51%
8 17% |
Causes of Cirrhosis:
HCV +
Alcohol
HBV +
HCV+ & Alcohol
NASH *
other
|
19 37%
9 16%
9 16%
2 3%
10 18%
5 9% |
20 42%
8 17%
7 15%
6 13%
2 4%
4 9 % |
a-fetoprotein (mg/dL) |
83.1 (1-1895) [1.1-163.3] |
45.5 (1.3-590) [11.4-79.5] |
N° of HCC nodules/ pts |
2.3 (1-6) [2-3] |
1.9 (1-8) [1-2] |
Largest lesion size (mm) § |
20.3 (10-60) [18.2-22.3] |
17.1 (10-70) [14-9-19.3] |
Patients bridging/downstaging º therapies |
29/5 62 % |
13/0 28% |
NPV of Imaging |
83.3% |
81% |
Recurrence of HCC |
4 7.2% |
4 8.5% |
Values in brackets represents range; values in square brackets represents 95% of Interval Confidence
* significant difference p=0.03;
§ significant difference p=0.04;
º significant more treated lesions in up-to-seven group p=0.0007
AFP levels were higher in the up-to-seven group (83.1 vs 45.5) but no signficant difference was noted.
No significant difference was noted regarding the overall three- and five-year survival between the two groups, with slightly better survival in the up-to-seven group (0.78 vs. 0.83 and 0.63 vs. 0.75) (Figure 2). In the Milan criteria group, 4/47 patients (8.5%) developed HCC recurrence: two patients had intra- and extrahepatic recurrence (lung and peritoneum) the others only liver recurrence. Among these patients three underwent bridging therapy with TACE and one downstaging therapy with TACE before LT. All patients with HCC recurrence died within three years.
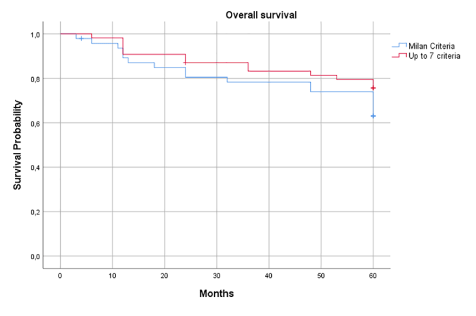
Figure 2. Overall survival of the two study groups
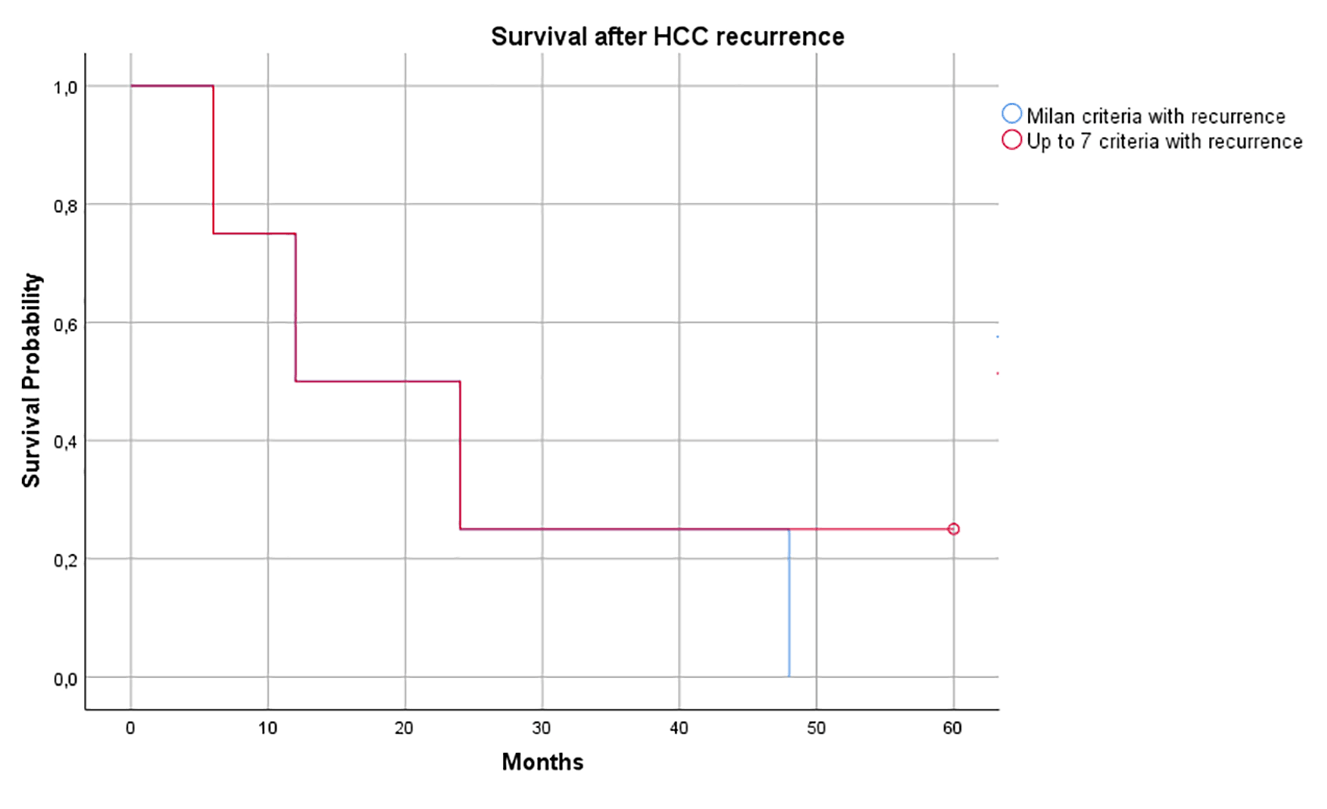
Figure 3. HCC survival rate of the two study groups
The up-to-seven group had also 4/54 (7.2%) patients with HCC recurrence in: one patients performed downstaging therapy with SIRT and subsequently TACE for a single lesion grater than 7 cm; another underwent bridging therapy with TACE for a lesion of 4.5 cm.; one more patients performed downs taging TACE fore multiple nodules and the last one had multple tiny HCC foci within the liver parenchyma
In the up-to-seven group 21/54 (38.9%) patients were considered beyond Milan Criteria at radilogical examination but within up-to-seven criteria.
Both groups reported a low survival rate after HCC recurrence at 24 months (25 %).
After comparing radiological and pathological findings, imaging underestimated HCC disease in nine cases (16.7%) in the up-to-seven group. In contrast, nine patients (19%) had a more advanced tumor disease than that reported at the radiological pre-transplant examination in the Milan criteria group. In four cases, the overestimation was related to a slightly larger size of the largest lesion out of the Milan criteria. In three patients, more lesions were discovered than those reported at imaging. In two cases, both of these conditions were observed. The NPV for each group was 84%,.
The possibility of safely increasing the selection criteria in HCC patients is of paramount importance, mainly in light of the growing incidence of HCC worldwide [8]. The impact of HCC in LT has even grown after the introduction in the clinical practice of downstaging techniques able to take back intermediate-stage HCC patients into transplantability criteria.
No significant differences were noted in terms of overall three- and five-year survival and HCC recurrence between patients transplanted with Milan criteria and up-to-seven criteria as the upper limit of transplantability. Interestingly, in the case of HCC recurrence, the up-to-seven group reported better results. In our study, the overall three- and five-year survival of patients transplanted with up-to-seven criteria is lower to that previously reported in the literature by Chan, et al. (87% and 82%) and higher compared to results published by Diaz, et al. (66,7% and 58.3%), Lei, et al. (77.8% and 74.6%) and de Ataide, et al. (74.5% and 65.3%) [9-12].
A recent increase in the number of interventional procedures performed has been observed in our experience, as documented by the significantly higher number of bridged/downstaged cases in the up-to-seven group.
Such a phenomenon is confirmed by several studies reported in the literature, in which the intention to safely expand the overall number of potentially transplantable patients encouraged more aggressive management during the waiting time [13-16].
HCC recurrence after transplantation remains a significant cause of graft loss and mortality, affecting up to 18% of recipients [17,18]. In our study, five-year HCC recurrence rates were 7.2% and 8.5% in the up-to-seven criteria and Milan criteria group, respectively. The reported data are significantly lower than those reported in the literature, being justified by proper patient selection and aggressive management during the waiting time.
Both groups had a low percentage of survivile at 24 months (25%) and in the Milan Criteria all patients died within 48 months. Unfortunately, this datum is in line with several reports, in which the clinical course after HCC recurrence post-LT is connected with poor survival [19].
Another critical aspect to underline in the present study is the leading role played by imaging in the pre-LT assessment of HCC and patient scheduling for LT. In the up-to-seven group, imaging underestimated the tumor disease in 9% of cases. In all the underestimated cases, downstaging treatments (SIRT and TACE) were performed. An overestimation was reported in 19% of cases in the Milan criteria group. Also in this case, all patients with misdiagnosed HCC underwent bridging therapies (TACE) before transplant.
The explanation for these results could be found in the difficulty of imaging (both CT and MR) in the evaluation of patients with multiple treated nodules. The application of mRECIST criteria after repeated treatments may be difficult, because the tumor tissue may persist after several treatments, even if it does not show enhancement during the arterial phase due to the inadequate arterial vascular supply. Application of response evaluation criteria in cancer of the liver (RECICL) should be a probably solution [20]. One more reason for imaging misdiagnosis could be related to diffuse and non-homogeneous enhancement of liver parenchyma after radioembolization persisting in the late vascular phases, or a large fibrous reaction masking the underlying lesions [21].
Moreover, the discovery of small foci of HCC, undetectable by imaging, is becoming more frequent in the case of multiple HCCs. Most likely, the appropriate imaging and clinical follow-up of these patients requires information obtained by tumor markers and morphological and functional imaging.
Patients scheduled with the up-to-seven criteria have a disease-free survival at three years of 65% compared to 74% of patients included in the Milan criteria group. This data should be considered acceptable, although slightly lower than that obtained using the Milan criteria [22].
Our study presents some limitations. Firstly, the groups in this study represent a small sample of the study population. Secondly, a limited number of patients in both the groups reported HCC recurrence. Thirdly the "up-to-seven" group include 20 patients who met "Milan criteria" Finally, we are not able to calculate how many patients dropped-out from the waiting lists over the course of the study.
The application of the up-to-seven criteria for LT demonstrates acceptable survival rates, and enable patients with more advanced HCC to undergo liver transplantation.
All authors have contributed equally to all stages of preparation of the article.
None.
None.
All authors have nothing to disclose.
- Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, et al. (1996) Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. New Engl J Med 334: 693-699. [Crossref]
- Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, et al. (2001) Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 33: 1394-1403. [Crossref]
- Decaens T1, Roudot-Thoraval F, Hadni-Bresson S, et al. (2006) Impact of UCSF criteria according to pre- and post-OLT tumor features: analysis of 479 patients listed for HCC with a short waiting time. Liver Transpl 12: 1761-1769. [Crossref]
- Mazzaferro V, Llovet JM, Miceli R, et al. (2009) Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 10: 35-43. [Crossref]
- https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS
- Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30: 52-60. [Crossref]
- International Working Party (1995) Terminology of nodular hepatocellular lesions. Hepatology 22: 983-993. [Crossref]
- Mazzaferro V, Sposito C, Zhou J, Antonio D Pinna, Luciano De Carlis, et al. (2018) Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for Hepatocellular Carcinoma. Gastroenterology 154: 128-139. [Crossref]
- de Ataide EC, Garcia M, Mattosinho TJ, Almeida JRS, Escanhoela CAF, et al. (2012) Predicting survival after liver transplantation using up-to-seven criteria in patients with hepatocellular carcinoma. Transplant Proc 44: 2438-2440. [Crossref]
- Chan SC, Fan ST, Chok KS, Cheung TT, Chan ACY, et al. (2011) Survival advantage of primary liver transplantation for hepatocellular carcinoma within the up-to-7 criteria with microvascular invasion. Hepatol Int 6: 646-656. [Crossref]
- León Díaz FJ, Pérez Daga JA, Sánchez Pérez B et al. (2016) Up-to-7 Criteria for Hepatocellular Carcinoma Liver Transplantation: A Retrospective Analysis of Experiences. Transplant Proc 48: 2969-2972. [Crossref]
- Lei JY, Wang WT, Yan LN (2013) Up-to-seven criteria for hepatocellular carcinoma liver transplantation: a single center analysis. World J Gastroenterol 19: 6077-6083. [Crossref]
- Schwartz M, Roayaie S, Uva P (2007) Treatment of HCC in patients awating liver transplantation. Am J of Transplant 7: 1875-1881. [Crossref]
- Mazzaferro V (2016) Squaring the circle of selection and allocation in liver transplantation for HCC: An adaptive approach. Hepatology 63: 1707-17071. [Crossref]
- Cillo U, Burra P, Mazzaferro V, Pinna AD, Spada M, et al. (2015) A Multistep, Consensus-Based Approach to Organ Allocation in Liver Transplantation: Toward a ‘‘Blended Principle Model’’. Am J Transplant 15: 2552-2556. [Crossref]
- Lopez PM, Villanueva A, Roayaie S, Llovet JM (2006) Neoadjuvant therapies for hepatocellular carcinoma before liver transplantation: a critical appraisal. Liver Transpl 12: 1747-1754. [Crossref]
- Duffy JP, Vardanian A, Benjamin E, Watson M, Farmer DG, et al. (2007) Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg 246: 502-511. [Crossref]
- Sotiropoulos GC, Molmenti EP, Losch C, Beckebaum S, Broelsch CE, et al. (2007) Meta-analysis of tumor recurrence after liver transplantation for hepatocellular carcinoma based on 1,198 cases. Eur J Med Res 12: 527-534. [Crossref]
- Mazzola A, Costantino A, Petta S, Bartolotta TV, Raineri M, et al. (2017) Recurrence of Hepatocellular Carcinoma After Liver Transplantation: An Update. Future Oncol 11: 2923-2293. [Crossref]
- Kudo M, Ueshima K, Kubo S, Sakamoto M, Tanaka M, et al. (2016) Response Evaluation Criteria in Cancer of the Liver (RECICL) (2015 Revised version). Hepatol Res 46: 3-9. [Crossref]
- Atassi B, Bangash AK, Bahrani A, Pizzi G, Lewandowski RJ, et al. (2008) Multimodality imaging following 90Y radioembolization: a comprehensive review and pictorial essay. Radiographics 28: 81-99. [Crossref]
- Majno P, Mazzaferro V (2006) Living donor liver transplantation for hepatocellular carcinoma exceeding conventional criteria: questions, answers, and demands for a common language. Liver Transpl 12: 896-898. [Crossref]
Editorial Information
Editor-in-Chief
Dr. Abdullah H. A. Almalki
Section Head of Nephrology, Department of Medicine, KAMC, Saudi Arabia
Article Type
Research Article
Publication history
Received date: April 06, 2021
Accepted date: April 20, 2021
Published date: April 23, 2021
Copyright
©2021 Martino MD. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Martino MD, Lai Q, Lucatelli P, Damato E, Calabrese A, et al. (2021) Comparison of Up-to-seven criteria with Milan Criteria for liver transplantation in patients with HCC. Trends in Transplant 14(3): DOI: 10.15761/TiT.1000300



