Abstract
Objective: Concomitant chemoradiation (RCT) represents the standard of care for locally-advanced nasopharyngeal carcinoma (NPC). Nevertheless, induction chemotherapy (IC) followed by RCT could be an interesting approach. Some trials showed a survival benefit of this therapeutic strategy, but it is not the standard of care. The aim of this study was to analyze clinical response after IC and to assess its impact on disease control and survival.
Materials and methods: We conducted a retrospective study from January 2008 to December 2014. Forty patients with locally advanced NPC were treated in our institute. All patients received IC with fluorouracyl-cisplatin (5 FU-CDDP) or fluorouracyl-cisplatin-Docetaxel (TPF) or Adriamycin-cisplatin (AD-CDDP). After IC, clinical response was evaluated, CCR was defined by a normal clinical and computed tomography examination. After IC, 14 patients received RCT and 22 patients received radiotherapy (RT) alone.
Results: Our study included 25 men and 15 women with a median age of 41years. Tumor was classified T1 in 5% of patients, T2 in 27%, T3 in 20% and T4 in 48%. 80% of patients had involved nodes (N+). Twenty patients received 5FU-CDDP, 16 received TPF and 4 received AD-CDDP. The occurrence of leucopenia was higher in the 5FU-CDDP (p=0.02) group. Gastrointestinal toxicity was higher in the TPF group (p=0.01). Anemia and thrombopenia were similar in the three groups. After IC, 18 patients (45%) achieved CCR, 7 of them had RCT and 11 had RT alone. 21patients (52%) achieved partial clinical response (PR) and 1 patient developed metastases. The CCR was higher in (5FU-CDDP) group (p>0.3). CCR followed by RCT was associated to better local control than RT alone. However, there was no benefit in overall survival in the CCR group compared to partial clinical response (PR).
Conclusion: Complete clinical response after IC followed by RCT in locally-advanced nasopharyngeal carcinoma is associated to a better local disease control without impact on survival.
Key words
induction chemotherapy, chemoradiotherapy, complete clinical response
Introduction
Nasopharyngeal carcinomas (NPC) are mainly represented by undifferentiated carcinoma nasopharyngeal type (UCNT). This cancer has an extremely unbalanced geographical distribution.
There are three levels of incidence: high 15-30 cases/100,000 inhabitants (Southern China, South East Asia), low 0.5-1/100000 inhabitants (Northern Europe, USA, Japan) and intermediate 3-12/100000 inhabitants in the Mediterranean Basin and North Africa. Tunisia has an intermediate incidence of NPC.
Epstein-Barr virus (EBV) represents a major risk factor of NPC and this tumor has substantial responsiveness to both radiotherapy and chemotherapy [1,2].
To decrease the rate of locoregional failure and the risk of distant metastases, treatment has substantially evolved in the last two decades from surgery through RT to multimodal chemoradiotherapy (RCT).
Current standard therapy for nasopharyngeal carcinoma (NPC) is platinum-based concurrent chemoradiation based on randomized data. However, until now conflicting data exist to support the addition of induction chemotherapy to RCT [3-5].
The aims of our retrospective study were to analyze the outcomes of a series of patients with locally advanced nasopharyngeal carcinoma (LA-NPC) treated with platinum based IC followed by RCT or RT, to evaluate the efficacy and toxicity of this regimen, and to study the prognostic value of complete clinical response for outcomes.
Materials and methods
It is a retrospective study conducted from January 2008 to December 2014 that included 40 patients with newly diagnosed histologically confirmed locally advanced NPC and treated in the SALAH AZAIZ Institute of oncology of Tunis, Tunisia.
Baseline imaging included bone scan, chest X-ray, abdominal ultrasound, head and neck contrast-enhanced computed tomography Patients were staged according to the American Joint Committee on Cancer TNM 2010 (7th edition). All tumors included were T3-T4 and/or N+ .All patients received induction chemotherapy .
Three induction chemotherapy regimens were used: fluorouracyl-cisplatin (5 FU-CDDP), fluorouracyl-cisplatin-Docetaxel (TPF) with primary prophylaxis with granulocyte colony-stimulating factor (G-CSF) and Adriamycin-cisplatin (AD-CDDP). Schedules of induction chemotherapy are summarized in Table 1.
Table 1. Schedules of induction chemotherapy.
|
IC schedules
|
Dose
|
|
fluorouracyl-cisplatin (5 FU-CDDP)
|
cisplatin 80 mg/m2 on day 1, 5-fluorouracil 1,000 mg/m2 as a continuous infusion on days 1-5
JI=J21
|
|
fluorouracyl-cisplatin-Docetaxel (TPF)
|
docetaxel 70 mg/m2 IV on day 1 plus cisplatin 75 mg/m2 IV on day 1 plus 5-FU 1000 mg/m2/day by continuous IV infusion on days 1-4
JI=J21
|
|
Adriamycin-cisplatin (AD-CDDP)
|
adriamycin (60 mg/m2) on day 1-cisplatine (100 mg/m2) on day 1
JI=J21
|
Patients suitable for treatment with a TPF induction chemotherapy were those with a good performance status, no contraindication to cisplatin or taxanes, and high tumor volume (T3/ T4/ N2/ N3). Other patients received (5 FU-CDDP) or (AD-CDDP) regimens.
Tumor response to induction therapy was evaluated before commencement of RCT or RT by physical examination, nasopharyngoscopy (NP) and head and neck computer tomography (CT).
Complete clinical response (CCR) was defined by a normal physical examination, normal NP and radiologic complete response. Radiological response was assessed using computed tomography images based on Response Evaluation Criteria in Solid Tumors (RECIST).
Then, 14 patients received concurrent chemoradiotherapy (RCT), 22 patients received radiotherapy (RT) alone. No patient received adjuvant chemotherapy. Three patients didn’t receive neither RCT nor RT because of deterioration of general status. One patient had metastatic progression and received salvage chemotherapy.
Three dimensional (3D) conformal radiation therapy was used. Primitive tumor and clinically positive lymph nodes received 70 Gy and prophylactic neck lymph node levels were delivered to 50 Gy in 2 Gy/fraction, once a day, five times a week.
After achievement of RCT, patients with a good performance status and good renal function received chemoradiotherapy. Patients with poor performance status or renal insufficiency received only radiotherapy . Schedules of RCT and RT are summarized in Table 2.
Table 2. Schedules of RCT and RT.
|
Schedule
|
Dose
|
|
chemoradiotherapy (RCT)
|
weekly cisplatin 40 mg/m2 and RT : Primitive tumor and clinically positive lymph nodes received 70 Gy and prophylactic neck lymph node levels were delivered to 50 Gy in 2 Gy/fraction, once a day, five times a week
|
|
Only radiotherapy (RT)
|
Primitive tumor and clinically positive lymph nodes received 70 Gy and prophylactic neck lymph node levels were delivered to 50 Gy in 2 Gy/fraction, once a day, five times a week
|
Results
From January 2008 to December 2014, 40 patients were assessed. This study included 25 men and 15 women. The median age was 41 years. 37.5℅ of patients were diagnosed with stage IV. Patient Characteristics are detailed in Table 3.
Table 3. Patients characteristics.
| |
N
|
%
|
|
Sex
|
|
Male
|
25
|
62.5
|
|
Female
|
15
|
37.5
|
|
Age
|
|
Years
|
41
|
|
|
Median Range
|
(11-63)
|
|
|
Median period of consultation
|
7,66 months (0-24)
|
|
UICC T-classification
|
|
T1
|
2
|
5
|
|
T2
|
11
|
27
|
|
T3
|
8
|
20
|
|
T4
|
19
|
48
|
|
UICC N-classification
|
|
N0
|
8
|
20
|
|
N1
|
7
|
18
|
|
N2
|
10
|
25
|
|
N3
|
15
|
37
|
|
M
|
|
|
|
M0
|
40
|
100
|
|
M1
|
0
|
0
|
|
UICC Stage
|
|
|
II
|
5
|
12.5
|
|
III
|
12
|
30
|
|
IV A
|
8
|
20
|
|
IV B
|
15
|
37.5
|
Histologically, all tumors were an undifferentiated carcinoma of nasopharynx (type III WHO).
Twenty patients received 5FU-CDDP, 16 received TPF and 4 received AD-CDDP. The median number of cycles of chemotherapy was three (2-4 cycles). The median period of treatment initiation was 1,72 months (0-8 months).Responses after induction chemotherapy are detailed in Table 4.
Table 4. Response after IC.
|
schedules of IC
Response after IC
|
5FU-CDDP
|
TPF
|
AD-CDDP
|
Total
|
|
Complete clinical response (CCR)
|
9(45℅)
|
7(43.75℅)
|
2
|
18(45%)
|
|
Partial clinical response (PR)
|
10
|
9
|
2
|
21(52.5%)
|
|
progression
|
1
|
0
|
0
|
1(2.5%)
|
|
Total
|
20
|
16
|
4
|
40 (100%)
|
In our study, complete clinical response was higher in (5FU-CDDP) group (p=0.3). The occurrence of leucopenia was higher in the 5FU-CDDP group (p=0.02) may be because patients received G-CSF in the TPF group. However, gastrointestinal toxicity was higher in the TPF group (p=0.01). Occurrence of anemia and thrombopenia were similar between treatment groups. 11℅ of registered toxicities were Grade 4 (more frequent with 5FU-CDDP).
After induction chemotherapy, 14 patients had RCT, 22 patients had only RT alone, 1 patient developed metastases and had salvage chemotherapy and 3 patients didn’t have neither RCT nor RT because of deterioration of general status. Treatment after induction chemotherapy is detailed in Table 5.
Table 5. Treatment after IC.
|
Treatment
Response after IC
|
RCT
|
RT alone
|
Salvage chemotherapy
|
No treatment
|
Total
|
|
CCR
|
7
|
11
|
0
|
0
|
18
|
|
PR
|
7
|
11
|
0
|
3
|
21
|
|
Progression
|
0
|
0
|
1
|
0
|
1
|
|
Total
|
14
|
22
|
1
|
3
|
40
|
In the RCT arm (14 patients), median number of chemotherapy cycles (weekly CDDP) was four (2-5 cycles). Primitive tumor and positive lymph nodes received 70 Gy and prophylactic neck lymph node levels were delivered to 50 Gy (2 Gy/fraction, once a day, five times a week).
In the RT arm (22 patients) median radiation dose of primitive tumor and positive lymph nodes was 70 Gy and prophylactic neck lymph node levels were delivered to 50 Gy. (2 Gy/fraction, once a day, five times a week). Complete response after RCT was 85℅and 77℅ after RT. Responses after RCT and RT are summarized in Table 6.
Table 6. Response to RCT and RT.
|
|
CCR after IC
|
PR after IC
|
|
Treatment after IC
|
RCT
|
RT
|
RCT
|
RT
|
|
N
|
7
|
11
|
7
|
11
|
|
Response
|
Remission: 6(85%)
Recurrence: 1(15%)
|
Remission :8(73%)
Recurrence: 3(27%)
|
Remission: 6(85%)
Recurrence: 1(15%)
|
Remission : 9(82%)
Recurrence : 2(18%)
2021 Copyright OAT. All rights reserv
|
Complete clinical response after induction chemotherapy followed by RCT was associated to better local control than in those who achieved CCR flowed by RT alone (85%vs 73%). Response to RCT was similar in the CCR and PR groups. However, RT was associated to better local control in PR group than CCR group. The median overall survival in the complete clinical response group after IC was: 38 months and was 30 months in partial response group (p>0.05).
There was no benefit of overall survival in the CCR group compared to PR (p=0.391). Comparison of OS between complete clinical response and partial response groups is showed in Figure 1.
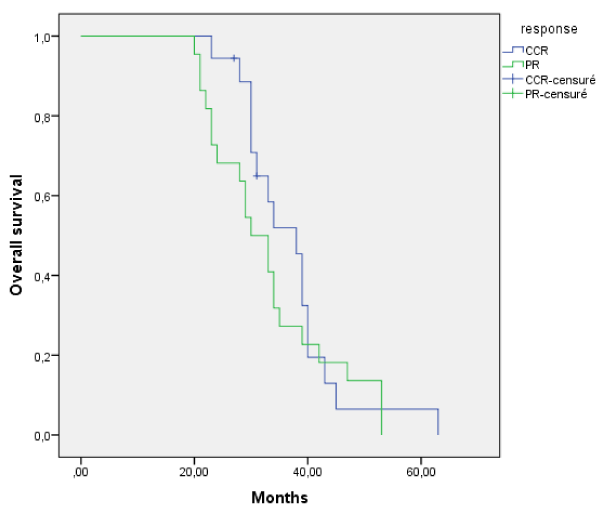
Figure 1. Comparison of overall survival between complete clinical response and partial response groups.
Discussion
Tunisia is an area of intermediate incidence of nasopharyngeal carcinoma (1-6 cases/100000) [6]. Therefore, defining the optimal treatment for patients with nasopharyngeal carcinoma is of the utmost importance. Treatment has substantially evolved in the last two decades. Current standard therapy for locally advanced (LA) nasopharyngeal carcinoma (NPC) is platinum-based concurrent chemoradiation.
Although many trials demonstrated a benefit of induction chemotherapy, this strategy is not yet the standard of care. In Tunisia, the majority of patients with LA-NPC in Tunisia received chemoradiation.
Epstein–Barr virus (EBV) is recognized as an etiologic agent of nasopharyngeal carcinoma [7]. Moreover, many studies considered human papilloma virus (HPV) as a cause for NPC [8]. In our study, EBV status was accessed in few number of patients making data unexpoitable. We didn’t access human papillomavirus status (HPV).
Until mid-1990, RT was the standard treatment for all stages of NPC. Definitive radiation without chemotherapy was associated to a significant risk of local recurrences [9].
In 1998, the phase III randomized intergroup study 0099 compared chemoradiotherapy to radiotherapy alone in patients with nasopharyngeal cancers. The investigational arm received chemotherapy with cisplatin 100 mg/m2 on days 1, 22, and 43 during radiotherapy; postradiotherapy, chemotherapy with cisplatin 80 mg/m2 on day 1 and fluorouracil 1,000 mg/m2/d on days 1 to 4 was administered every 4 weeks for three cycles. This trial concluded that chemoradiotherapy is superior to radiotherapy alone for patients with advanced nasopharyngeal cancers with respect to PFS and overall survival [10].
Another Phase III randomized trial conducted in Singapore between September 1997 and May 2003 showed that RCT followed by adjuvant chemotherapy in LA-NPC improved significantly distant metastasis control, disease free survival (DFS) and overall survival (OS) compared to RT alone [11].
A systematic review and meta-analysis of randomized trials (1753 patients) conducted in 2006 by Baujat and al compared cisplatin-based chemotherapy plus RT with RT alone in locoregionally advanced NPC. This meta-analysis concluded that patients receiving any combined modality therapy (neoadjuvant, concurrent, or adjuvant chemotherapy) have an absolute event- free-survival (EFS) and OS benefit with the highest benefit resulting from RCT (The pooled hazard ratio of death was 0.82 (95% confidence interval, 0.71-0.94; p=0.006 and pooled hazard ratio of tumor failure or death was 0.76 (95% confidence interval, 0.67-0.86; p<0.0001) [12].
Radio-chemotherapy was considered as the standard of care as is the case in Tunisia. In order to improve survival and disease control, many trials evaluated the induction chemotherapy followed by RCT. Toxicity was also assessed. These studies produced mixed results.
In 2004, a meta-analysis of Langendijk and al concluded that the addition of neoadjuvant chemotherapy to radiation resulted in a significant reduction (P=0.005) of the incidence of locoregional recurrences (relative risk: RR, 0.74; 95% CI, 0.60 to 0.91) and significant reduction (P=0.0003) of the incidence of distant metastases, with an RR of 0.67 (95% CI, 0.54 to 0.83) without OS benefit (p=0.13). There was no benefit to add adjuvant chemotherapy [13]. The study of J L OH and al demonstrated that IC followed by RCT in LA NPC (3cycles of IC consisting of cisplatin, 5FU, leucovorin and interferon-α2b were administered, followed by RCT consisting of 7 cycles of 5-FU, hydroxyurea and once-daily RT on a week-on week-off schedule) resulted in excellent overall survival at 3 and 5 years (respectively 88% and 77%). Progression-free survival (PFS) at 3 and 5 years was respectively 92% and 86% with acceptable toxicity .After IC, there were 54.2% of complete response and 45.8% of partial response. After RCT, there was 100℅ of complete response. At 5 years, actuarial locoregional control was 93% and actuarial distant control 92% [14].
In a randomized phase II trial, Hui et al. reported that neoadjuvant docetaxel-cisplatin followed by RCT provided a 3-year overall survival benefit in stage III-IVB NPC compared to RCT alone (94.1% vs 67.7%, p=0.012). Acute and late toxicities and quality of life scores were comparable [15].
In our series, complete response after induction chemotherapy was 45% versus 54,2% in the series of J L OH et al. RCT offered more remission than RT alone (85% VS 77%) which is similar to previous studies. The type of response after IC didn’t affect OS.
In contrast, there are negative studies. A greek phase II trial randomized 141 patients with LA-NPC to either 3 cycles of IC with cisplatin, epirubicin, and paclitaxel followed by definitive RT with concurrent weekly cisplatin versus RCT alone. There was no difference in the number of patients who completed radiation. There was no significant difference between the two treatment groups in OS (67% versus 72%, p=0.65) and PFS (65% versus 64%, p=0.71) [16].
The trial NPC-0501 evaluated the therapeutic gain by changing from concurrent-adjuvant to induction-Concurrent chemoradiotherapy, changing From Fluorouracil to Capecitabine, and changing from conventional to accelerated radiotherapy fractionation in patients with LA-NPC. Preliminary results indicated that the benefit of changing to an induction-concurrent sequence remains uncertain. Replacing fluorouracil with oral capecitabine warrants further validation in view of its convenience, favorable toxicity profile, and favorable trends in efficacy. Accelerated fractionation was not recommended for patients with locoregionally advanced NPC who receive chemoradiotherapy [17]. In our study three regimens of IC were used. In the literature many trials compared different molecules and protocols of induction chemotherapy followed by RCT. The efficacy of docetaxel-cisplatin (TP) as induction chemotherapy regimen in NPC was similar to that of 5 -fluoruracil –cisplatin (PF) regimen, and the adverse events were tolerable [18].
Han SH and al demonstrated that the efficacy of vinorelbine-cisplatin (NP) regimen induction chemotherapy plus concurrent chemordiotherapy for advanced NPC is similar to that of TP regimen. The 3-year overall survival rates, disease-free-survival rates, locoregional relapse-free survival rates and distant metastasis-free survival rates in the NP and TP groups were 84.2% and 82.9%, 71.1% and 74.3%, 89.5% and 91.4%, 81.6% and 77.1%, respectively (P>0.05). The toxicity of the NP regimen is lower than that of TP regimen [19]. In our study, NP regimen wasn’t used. Several trials showed a benefit of TPF regimen. A phase II study showed that TPF induction chemotherapy followed by RCT had promising activity with manageable toxicity. The 3-year progression-free survival was 75.6% and the 3-year overall survival was 86.1% [20].
According to Wen-Fei Li and al, the addition of TPF induction chemotherapy to RCT significantly increased failure-free survival, overall survival, and distant failure-free survival rates [21].
A Chinese study showed that IC followed by RCT was an effective treatment strategy for LA-NPC.
Induction chemotherapy with TPF conferred satisfactory long-term survival and slightly improved PFS (77.0% vs. 73.5%; P=0.510) and OS (80.7% vs. 77.9%, P=0.446) as compared with the classic PF regimen, and toxicity was tolerable [22].
In our study, patients who received induction 5FU-CDDP regimen achieved higher complete clinical response rate compared with that of patients receiving TPF regimen probably because there were more stage IVA –IVB NPC in the TPF group.
In order to refine indications of induction chemotherapy, prognostic factors and risk stratification could help. Response to induction chemotherapy may have potential clinical value.
Liu and al. revealed that the unsatisfactory tumor response after induction chemotherapy (stable disease or disease progression) could predict poor prognosis for patients with advanced-stage NPC (locoregional relapse-free survival, PFS) [23]. However, another trial concluded that the overall tumor response after induction chemotherapy was an independent prognostic factor for disease-free-survival, OS and locoregional recurrence free survival [24].
Conclusion
Induction chemotherapy followed by chemoradiation in LA-NCP carcinoma offers encouraging results. It doesn’t be until today a standard of care. Complete clinical response after induction chemotherapy could be an interesting prognostic factor. This therapeutic strategy should be explored further in randomized settings in order to establish prognostic factors and to identify which patients benefit the most from induction chemotherapy followed by RCT.
References
- Boussen H, Bouaouina N (1999) Cancer du nasopharynx (cavum) (1999). Oto-rhinolaryngologie: 23.
- Boussen H, Bouaouina N, Mokni-Baizig N, Gamoudi A, Chouchane L, et al. (2005) Nasopharyngeal carcinoma. Recent data. Pathol Biol (Paris) 53: 45-51. [Crossref]
- Golden DW, Rudra S, Witt ME, Nwizu T, Cohen EE et al. (2013) Outcomes of induction chemotherapy followed by concurrent chemoradiation for nasopharyngeal carcinoma. Oral Oncol 49:277-282. [Crossref]
- Peng H, Chen L, Zhang J, Li WF, Mao YP, et al. (2017) Induction Chemotherapy Improved Long-term Outcomes of Patients with Locoregionally Advanced Nasopharyngeal Carcinoma: A Propensity Matched Analysis of 5-year Survival Outcomes in the Era of Intensity-modulated Radiotherapy. J Cancer 8: 371-377. [Crossref]
- OuYang PY, Bi ZF, Zhang LN, You KY, Xiao Y, et al. (2016) Outcomes of Induction Chemotherapy Plus Intensity-Modulated Radiotherapy (IMRT) Versus IMRT Plus Concurrent Chemotherapy for Locoregionally Advanced Nasopharyngeal Carcinoma: A Propensity Matched Study. Transl Oncol 9: 329-335. [Crossref]
- Karray H, Ayadi W, Fki L, Hammami A, Daoud J, et al. (2005) Comparison of three different serological techniques for primary diagnosis and monitoring of nasopharyngeal carcinoma in two age groups from Tunisia. J Med virolgoy 9: 1-10. [Crossref]
- Mould RF, Tai TH (2002) Nasopharyngeal carcinoma: treatments and outcomes in the 20th century. Br J Radiol 75: 307-339. [Crossref]
- Singhi AD, Califano J, Westra WH (2012) High-risk human papillomavirus in nasopharyngeal carcinoma. Head Neck 34: 213-218. [Crossref]
- Cooper JS, del Rowe J, Newall J (1983) Regional Stage IV carcinoma of the nasopharynx treated by aggressive radiotherapy. Int J Radiat Oncol Biol Phys 9: 1737-1745. [Crossref]
- Al-Sarraf M, Leblanc M, Giri S, Fu KK, Cooper J, et al. (1998) Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup Study 0099. J ClinOncol 16: 1310-1317. [Crossref]
- Wee J, Tan EH, Tai BC, Wong HB, Leong SS, et al. (2005) Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol 23: 6730-6738. [Crossref]
- Baujat B, Audry H, Bourhis J, Chan AT, Onat H, et al. (2006) chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data metaanalysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Bio lPhys 64: 47-56. [Crossref]
- Langendijk JA, Leemans CR, Buter J, Berkhof J, Slotman BJ (2004) The additional value of chemotherapy to radiotherapy in locally advanced nasopharyngeal carcinoma: a meta-analysis of the published literature. J Clin Oncol 22: 4604-4612. [Crossref]
- Oh JL, Vokes EE, Kies MS, Mittal BB, Witt ME, et al. (2003) Induction chemotherapy followed by concomitant chemoradiotherapy in the treatment of locoregionally advanced nasopharyngeal cancer. Ann Oncol 14: 564-569. [Crossref]
- Hui EP, Ma BB, Leung SF, King AD, Mo F, et al. (2009) Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol 27: 242-249. [Crossref]
- Fountzilas G, Ciuleanu E, Bobos M, Kalogera-Fountzila A, Eleftheraki AG, et al. (2012) Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: a randomized phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluation. Ann Oncol 23: 427-435. [Crossref]
- Lee AW, Ngan RK, Tung SY, Cheng A, Kwong DL, et al. (2015) Preliminary results of trial NPC-0501 evaluating the therapeutic gain by changing from concurrent-adjuvant to induction-concurrent chemoradiotherapy, changing from fluorouracil to capecitabine, and changing from conventional to accelerated radiotherapy fractionation in patients with locoregionally advanced nasopharyngeal carcinoma. Cancer 121: 1328-1338. [Crossref]
- Xie FY, Qi SN, Hu WH, Zou GR, Peng M, et al. (2007) Comparison of efficacy of docetaxel combined cisplatin (TP regimen) and cisplatin combined 5-fluorouracil (PF regimen) on locally advanced nasopharyngeal carcinoma. Ai Zheng 26: 880-884. [Crossref]
- Han SH, Yu L, Zhang Z, Zhang PJ, Song HP, et al. (2013) Evaluation of induction chemotherapy with vinorelbine plus cisplatin (NP) or docetaxel plus cisplatin (TP) combined with concurrent chemoradiotherapy for patients with locally advanced nasopharyngeal carcinoma. Zhonghua Zhong Liu Za Zhi 35: 623-626.[Crossref]
- Bae WK, Hwang JE, Shim HJ, Cho SH, Lee JK, et al. (2010) Phase II study of docetaxel, cisplatin, and 5-FU induction chemotherapy followed by chemoradiotherapy in locoregionally advanced nasopharyngeal cancer. Cancer Chemother Pharmacol 65: 589-595. [Crossref]
- Li WF, Chen L, Sun Y, Ma J (2016) Induction chemotherapy for locoregionally advanced nasopharyngeal carcinoma. Chin J Cancer 35: 94. [Crossref]
- Huang Z, Feng J, Li S, Wei W, Zhang Get al. (2013) Is the Neoadjuvant Docetaxel, Cisplatin and 5-Fluorouracil regimen Superior to Classic Cisplatin and 5-Fluorouracil for locoregionally Advanced Nasopharyngeal Carcinoma? J Can Res Updates 2: 297-305.
- Liu LT, Tang LQ, Chen QY, Zhang L, Guo SS, et al. (2015) The Prognostic Value of Plasma Epstein-Barr Viral DNA and Tumor Response to Neoadjuvant Chemotherapy in Advanced-Stage Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys 93: 862-869. [Crossref]
- Peng H, Chen L, Zhang Y, Li WF, Mao YP (2016) The Tumour Response to Induction Chemotherapy has Prognostic Value for Long-Term Survival Outcomes after Intensity-Modulated Radiation Therapy in Nasopharyngeal Carcinoma. Sci Rep 6: 24835. [Crossref]

