Abstract
Sinus of Valsalva aneurysm (SOVA) is an anomalous aortic root enlargement that occurs between the aortic valve annulus and the sinotubular junction. SOVA rupture is a rare and potentially fatal cardiac anomaly that requires prompt attention. A timely diagnosis and appropriate management of the latter are usually necessary for the procurement of a favorable prognosis. Whereas right coronary sinuses are usually most commonly affected followed by non-coronary sinuses, sinus of Valsalva aneurysms rarely arise from the left coronary sinus due to external support from the right ventricle and the pulmonary trunk. Herein, we present a 62-year-old Caucasian male who initially presented with severe dyspnea, and was noted to have a contained, ruptured sinus of Valsalva aneurysm arising from the left coronary sinus. Diagnosis was made using different imaging modalities, and he was subsequently managed and treated promptly with a successful outcome. Given its rarity and potential for serious comorbidities and mortality, we aim to provide a review of SOVA rupture epidemiology, diagnosis, and management.
Keywords
Aortic neurysm, Aortic Rupture, echocardiography, Computed Tomography Angiography (CTA), Sinus of Valsalva, Contained, Case Report
Introduction
Sinus of Valsalva aneurysm (SOVA) is an anomalous aortic root enlargement that occurs between the aortic valve annulus and the sinotubular junction. SOVA rupture is a rare life-threatening cardiac anomaly that requires immediate intervention [1,2]. Aneurysm rupture can cause acute onset of symptoms, including dyspnea, and if not immediately addressed, can ultimately compromise cardiac output and cause rapid decline in patient status. Etiology of SOVA can be either acquired or congenital [1-5]. Whereas cases of SOVA rupture are well reported in the literature, contained ruptures are seldom described. Herein, we present a case of a patient who presented with a contained rupture of SOVA that precipitated from non-infective endocarditis.
Case Presentation
A 62-year-old Caucasian male with a medical history of essential hypertension, deep venous thrombosis, on oral anticoagulant, and obesity presented to our facility due to sudden onset moderate substernal chest pain exacerbated upon exertion and associated with severe dyspnea, requiring supplemental oxygen. His blood pressure was 126/56 mmHg and his pulse rate were 92 beats per minute. The patient was noted to have a 3/6 pansystolic murmur loudest in the aortic region and radiating to the neck. Crackles were noted in the left lower lung lobe. Initial troponin was 0.679 ng/mL and N-terminal prohormone of brain natriuretic peptide (NT-proBNP) 14,058 pg/mL. Other pertinent laboratory findings are noted in [Table 1]. Electrocardiogram (ECG) revealed sinus rhythm with premature supraventricular complexes, nonspecific ST changes. He was administered full-strength aspirin, heparin drip was initiated, and he was admitted to the cardiovascular service. Chest radiograph revealed findings consistent with pulmonary edema, otherwise was largely unremarkable. Venous doppler lower extremity ultrasound was negative for deep venous thrombosis (DVT) and computed tomography angiography (CTA) of the chest was negative for pulmonary embolism (PE) but revealed findings suggestive of mild congestive heart failure with moderate bilateral pleural effusions [Figure 1].
Table 1. Pertinent laboratory findings on admission
Tests |
Results |
References |
Units |
White blood count |
8.8 |
4.0 - 10.5 |
10³ uL |
Hemoglobin |
8.4 |
13.7 - 17.5 |
g/dL |
Hematocrit |
27.5 |
40.1 - 51 |
% |
MCV |
85.1 |
79.0 - 92.2 |
fl |
ESR |
62 |
0 - 10 |
mm/hr |
CRP |
11.2 |
0 - 0.29 |
mg/dL |
Troponin |
0.679 |
0 - 0.045 |
ng/mL |
NT-Pro-BNP |
14058 |
0 - 900 |
pg/mL |
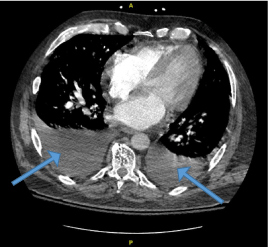
Figure 1. CT chest demonstrating moderate bilateral pleural effusion suggestive of mild CHF (blue arrows)
CTA of the aorta was performed, which revealed a 4.6 cm x 3.5 cm saccular outpouching along the posterior aspect of the aortic root and sinuses of Valsalva. These findings were consistent with a sinus of Valsalva aneurysm, which appeared to arise from the left coronary sinus. Imaging study was negative for aortic dissection. The patient then underwent a transesophageal echocardiogram (TEE), which demonstrated a tri-leaflet aortic valve with thick echodensities strongly suggestive of vegetations. TEE further revealed dilatation of the left sinus of Valsalva [Figure 2], severe aortic insufficiency, mild mitral and tricuspid regurgitation. Coronary angiogram was consistent with moderate non-obstructive coronary artery disease.
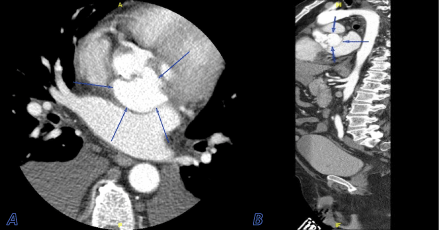
Figure 2. Cross-sectional view (A) and coronal view (B) of a CTA of the aorta demonstrating a 4.6 cm x 3.5 cm saccular outpouching along the posterior aspect of the aortic root and sinuses of Valsalva (blue arrows)
Broad-spectrum antibiotic therapy was initiated, heparin drip was discontinued, and cardiothoracic surgery was consulted for emergent surgical intervention. Aortic root and proximal ascending aorta replacements were performed via a mini sternotomy approach without complications. Intraoperative findings revealed a perforated posterior aorta along the non-coronary and left coronary cusps, with contained rupture onto the dome of the left atrium. The patient tolerated the surgery well and was transferred to the Cardiovascular Intensive Care Unit in stable condition. Final blood culture was negative, and he was later discharged home with home health care. The aortic valve pathology was positive for cluster of differentiation (CD) 68 supporting macrophage aggregation consistent with reaction to adjacent tissue injury.
Discussion
SOVA is a rare cardiac anomaly [2,4]. Approximately 0.09-0.15% of the general population is estimated to have SOVA, based on a large autopsy series [1,5]. This condition is 3-4 times more commonly found in males, with a higher incidence in men of Asian descent [6]. Congenital aneurysms result from localized weakness of the elastic lamina between the aortic media and the annulus fibrosus of the aortic valve. Congenital causes are more common and are typically associated with connective tissue disease that affects aortic wall musculature, including Marfan’s syndrome and Ehlers-Danlos syndrome. Valvular disease including bicuspid aortic valve is also associated with congenital SOVA. Acquired SOVA is commonly linked to infectious causes, including syphilis, tuberculosis, and infective endocarditis [7]. Atherosclerotic changes with age, injury from trauma, aortic valve replacement, and vasculitides are also common culprits of SOVA [1,3,4] [Figure 3].
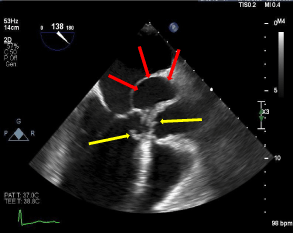
Figure 3. Parasternal long access view of bedside TEE, demonstrating thick aortic valve echodensities, suggestive of vegetations (yellow arrows), and left coronary sinus of valsalva (red arrows)
It has been documented that right coronary sinuses are usually most commonly affected (65-85%), followed by non-coronary sinus (10-30%), with only less than 5% in the left coronary sinus [8]. Sinus of Valsalva aneurysm rarely arises from the left coronary sinus due to external support from the right ventricle and the pulmonary trunk [1]. When SOVA is suspected, it is vital to identify its location because patient symptoms are linked to the size of the aneurysm, the site of rupture, and the speed at which the rupture occurs. Rupture of the right and noncoronary sinuses can lead to a left to right shunting of blood by forming a communication between the aorta and either the right atrium or the right ventricular outflow tract [2]. This ultimately can cause severe hypoxia, decreased cardiac output, and severe right heart overload. Left coronary SOVA rupture as seen in this patient, however, can be clinically less significant as it causes communication with the left atrium or the left ventricular outflow tract, keeping the heart’s hemodynamics relatively unchanged. Due to its less dangerous clinical presentation, a left coronary SOVA rupture can be present chronically, and only causes significant symptoms when superimposed with another condition that interferes with cardiac hemodynamics, such as deteriorating valvular disease, endocarditis, pulmonary embolism, and myocardial ischemia [1,4] [Figure 4].

Figure 4. TEE demonstrating ruptured aortic root at the level of the left sinus of Valsalva (red arrows)
In this case, the pathology result of the aortic valve vegetations was negative for infectious etiology, however, was positive for CD68 macrophage aggregation. It is assumed that this presentation could have been multifactorial as a consequence of ruptured aortic root releasing macrophages, or due to a prolonged state of hypoxia, inducing a hypercoagulable state, also leading to macrophage release which can further damage the aortic valves [9,10].
Regardless of the location or etiology, SOVA can potentially be life-threatening. SOVA is usually asymptomatic, but can sometimes present with chest pain, dyspnea, palpitations, shortness of breath, or syncope [2,3,7]. Intact SOVA can cause occlusion of coronary artery perfusion and aortic insufficiency which can present as an acute coronary syndrome and can also subsequently lead to congestive heart failure. Furthermore, both ruptured and non-ruptured SOVA can significantly compromise the aortic valve, causing severe aortic regurgitation which can often elicit symptoms. Larger SOVAs can also serve as a nidus for thrombus formation, causing further complications. In the rare event of a contained, ruptured SOVA, it is considered a life-threatening complication that, if delayed in diagnosis, can lead to a poor prognosis[Figure 5].
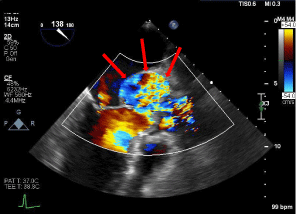
Figure 5. TEE with Doppler revealing left coronary sinus of valsalva, returning to the left ventricle (red arrows)
On chest auscultation, findings can be notable for a continuous murmur or a diastolic decrescendo murmur consistent with aortic regurgitation. Cine cardiac MRI is considered the gold standard for the diagnosis of a Sinus of Valsalva aneurysm [1], however, it is seldom required as other imaging modalities such as echocardiography and CT imaging are usually synergistically sufficient and efficient at providing timely diagnosis and pertinent details [1,7,11]. In our case, the patient initially underwent CT imaging, followed by transthoracic and transesophageal echocardiography, which provided sufficient pertinent details leading to diagnosis.
Coronary angiography is usually performed prior to cardiac surgery for assessment of possible coronary bypass grafting in patients with high-risk for having coronary artery disease. The most favorable management of an asymptomatic, intact aneurysm is unclear [1,5]. In fact, Martin et al. reported a case of a patient with congenital left coronary SOVA, who was followed for 19 years without surgical intervention or illness progression [2,12]. Ruptured SOVA, however, warrants urgent cardiovascular surgical evaluation for a prompt intervention to prevent clinical deterioration. The mean survival in patients who have not undergone surgical repair after a ruptured SOVA is about four years [5,6]. The prognosis after the surgical repair of SOVAs is satisfactory, with documented survival rates close to 94% at 10 years and 90% at 15 years [3,5,6,13].
This case illustrates the importance of timely diagnosis of a contained ruptured left SOVA which is a very rare, underreported, and dangerous condition that can rapidly deteriorate and lead to severe cardiac morbidities, including death. A high index of suspicion is required for appropriate workup and management because it can present with a myriad of clinical presentations, most of which can readily point clinicians towards other more common conditions, such as acute coronary syndrome, pulmonary embolism, and pneumonia amongst others, while their management can be completely different and conflicting. Ruptured SOVA can escalate to multiple cardiac ramifications, including cardiac tamponade, hemodynamic compromise, and left ventricular outflow tract obstruction, all of which can lead to a dire prognosis.
Conclusion
In this article, we have presented a rare case of contained ruptured left coronary SOVA, noted on imaging and confirmed intraoperatively. SOVAs can have a variety of clinical presentations, which can easily overlap with more common causes, therefore a high level of suspicion is needed to guide appropriate investigations for a timely diagnosis and management. Whether symptomatic or not, any ruptured SOVA always necessitates surgical repair.
Conflict of interest
The authors have no conflict of interest.
References
- Bass D, Tivakaran VS (2021) Sinus of Valsalva aneurysm. StatPearls. [Crossref]
- Moustafa S, Mookadam F, Cooper L, Adam G, Zehr K, et al. (2007) Sinus of Valsalva aneurysms- 47 years of a single center experience and systematic overview of published reports. Am J Cardiol 99: 1159-1164. [Crossref]
- Mohammed R, Dandekar U (2021) Large sinus of Valsalva aneurysm presenting as acute coronary syndrome. Asian Cardiovasc Thorac Ann 29: 411-413. [Crossref]
- Moreels S, Dymarkowski S, De Ridder S (2012) Ruptured sinus of valsalva in an asymptomatic patient-A case report. Eur Cardiol 8: 139-141.
- Post MC, Braam RL, Groenemeijer BE, Nicastia D, Rensing BJ, et al. (2010). Rupture of right coronary sinus of Valsalva aneurysm into right ventricle. Neth Heart J 18: 209-211. [Crossref]
- Yan F, Huo Q, Qiao J, Murat V, Ma SF (2008) Surgery for sinus of valsalva aneurysm: 27-year experience with 100 patients. Asian Cardiovasc Thorac Ann 16: 361-365. [Crossref]
- Mørk SR, Eftekhari A, Tang M, Klaaborg KE (2019) Giant unruptured aneurysm of the left coronary sinus of Valsalva presenting as acute coronary syndrome: a case report. Eur Heart J Case Rep 3: ytz053 [Crossref]
- Afshar AH, Kolesnikov S, Pourafkari L, Nader ND (2015) Right Valsalva sinus aneurysm protruding into the right ventricle: a case report. J Cardiovasc Thorac Res 7: 126-128. [Crossref]
- Khazen W, M'bika JP, Tomkiewicz C, Benelli C, Chany C, et al. (2005) Expression of macrophage-selective markers in human and rodent adipocytes. FEBS Lett 579: 5631-5634. [Crossref]
- Deodhar AK, Rana RE (1997) Surgical physiology of wound healing: a review. J Postgrad Med 43: 52-56. [Crossref]
- Wang KY, Sutton MSJ, Ho HY, Ting CT (1997) Congenital sinus of Valsalva aneurysm: a multiplane transesophageal echocardiographic experience. J Am Soc Echocardiogr 10: 956-963. [Crossref]
- Martin LW, Hsu I, Schwartz H, Wasserman AG (1986) Congenital aneurysm of the left sinus of Valsalva: report of a patient with 19-year survival without surgery. Chest 90: 143-145. [Crossref]
- Takach TJ, Reul GJ, Duncan JM, Cooley DA, Livesay JJ, et al. (1999). Sinus of Valsalva aneurysm or fistula: management and outcome. Ann Thorac Surg 68: 1573-1577. [Crossref]





