Introduction: The Hedgehog (Hh) molecular pathway is especially important during embryonic development. Later in life it can affect the process of carcinogenesis. Specifically, in colon cancer, two ways of influencing the pathway are proposed, either with a paracrine role, where it creates a microenvironment conducive to carcinogenesis, or with its autocrine activation, promoting metastasis.
Methods: In tissue of 53 patients, the expression of the IH index was estimated by immunohistochemistry and correlated with age, sex, tumor location and the presence of distant metastases.
Purpose: The aim of this study is to highlight the importance of the IHH index in the course of the disease either as a predictor or as a future therapeutic goal.
Results: The expression of the HH surface marker does not appear to be affected by the location of the tumor in the large intestine, similarly sex and age are independent elements, which are not related to the most common or not occurrence, of the above group of cancer stem cells. Essentially, however, the occurrence of distant metastases is statistically significant with p = 0.004, in patients with a higher incidence of HHH (+) cells, which makes their presence associated with a more aggressive form of the disease.
Discussion: It is clear that IHH (+) stem cells have a negative prognoses for patients with colorectal adenocarcinoma. They are associated with a more aggressive form, as the occurrence of metastases is more common in a statistically significant percentage compared to IHH (-) patients. This means that therapeutic targeting of these cells is a need for more desirable therapeutic results.
colorectal, stem, cells, hedjehog
The Hedgehog (Hh) molecular pathway is especially important during embryonic development. Later in life it can favor the process of carcinogenesis (Figure 1). Most basal cell carcinomas are characterized by mutations that lead to autonomic pathway activation [1]. Specifically, in colon cancer, 2 ways of influencing the pathway are proposed, either with a paracrine role, where it creates a microenvironment conducive to carcinogenesis, or with its autocrine activation, promoting metastasis [2].
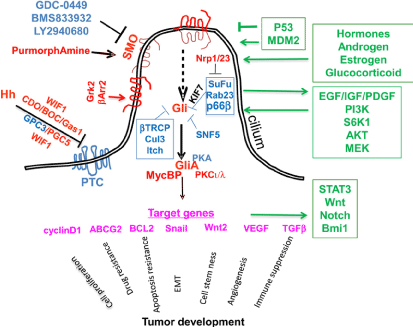
Figure 1. Diagram of the Hh pathway in mammals. Smo is the key to signal switching. In the absence of ligases, the PTC receptor is placed in specific protrusions of the eukaryotic cell, where it inhibits SMOs. Hh co-receptors are CDO (cell adhesion molecule), Gas1, Glypican 3 and 5. The WNT inhibitor (WINF1) also regulates Hh signaling through co-receptors. Gli proteins, through other agents (Su / Fu / KIF7) take a form that leads to pathway suppression. There are several negative regulators of Gli, such as (Rab23, protein kinase A, tumor suppressant SNF5, Culin3, p66β, Itchy E3 ubiquitin ligase). Presence Hh PTCs cannot suspend SMOs. Initial placement of SMOs requires β-arrestin 2 and a G protein kinase receptor. Once the Glis become active, they will activate specific target genes. KIF, SuFu agents can repress this sequence. The positives are red and the negatives are blue. The paths that interact with Hh are green. The lack of lashes (where SMOs are placed on cells) in mice protects them from developing basal cell carcinoma, while in many cases they are also absent from human cancer cells [7]
Differentiated enterocytes secrete Indian Hedgehog (Ihh) ligase, which binds to the inhibitory receptor Patched 1 (Ptch1), leading to a decrease in the activity of the Smoothened receptor (Smo), resulting in the initiation of a signal sequence that activates the proteases associated with oncogene (Gli). The expression of these proteins is the most reliable indicator of molecular pathway activity [3].
Smo antagonists have recently been used in the treatment of basal cell carcinomas [4]. In trials also of patients with pancreatic and colon cancer to date, the results have been lower than expected [5,6] (Figure 1).
Study [8] investigated Hh levels in patients diagnosed with colon cancer. It was therefore found that the presence of Hh ligases was not proportional to the levels of Gli proteins. In several cases with KRAS mutation, there has been an increase in Gli1 [9]. However, no association was found between KRAS mutants and Gli1 mRNA. However, there was a weak correlation between APC mutation and low Gli1 levels. There is a different effect on the disease from the ligases in the layer with those directly related to the tumor cells.
Gorlin syndrome affects the development of basal cell carcinomas in various parts of the body. In patients with this syndrome, increased expression of Hh target genes was found without detecting mutations. In fact, the increased activity of the path concerned either the volume directly or the layer [10]. An increased incidence of gastrointestinal stromal tumors has also been found in a study with PTCH1 deletion, indicating an association between IHH and GIST [11]. There are, of course, cases that contradict the data so far, such as a study where the expression of the oncogenic molecule SmoM2 had no effect on KRAS-induced carcinogenesis in the pancreas and prostate [12,13]. However, these data do not reverse the fact that the IHH pathway is involved in metastasis, a process that significantly alters patients' prognosis.
More and more evidence are coming to support the view of the importance of IHH in the function of cancer stem cells. For example, leukemia stem cell proliferation is directly dependent on IHH [14]. On the other hand, the effect of the molecular pathway on hematopoietic stem cells has not yet been clarified and there is disagreement in scientific opinions [14,15]. According to the model of cancer stem cells, pathway activation imparts chemoresistance and radiation resistance to the tumor [16]. In a study of pancreatic cancer, the use of an inhibitor of IHH was found to enhance the delivery of the chemotherapeutic drug [17]. However, it has not been proven whether pathway activation has a positive or negative effect on pancreatic cancer, as there are studies with conflicting results [18,19].
Chemotherapy and radiotherapy are the mainstays of cancer treatment. For example, overexpression of MAP3K10 enhances the proliferative potential of pancreatic cancer cells and reduces tumor sensitivity to chemotherapy through increased Gli1,2 expression [20]. Intersection of the IHH and PI3K / AKT pathways has been reported in prostate and lung cancer, and a combination of IHH and Notch inhibitors has been tested [21,22]. Elevated IHH levels lead to an increase in Bcl2, resulting in an increased rate of cancer cell proliferation and resistance to apoptotic mechanisms [23]. Therefore, the action of the pathway on chemoresistance varies in different types of cancer and is related to the function and levels of other molecular pathways.
However, the IHH pathway also plays a role in the metastatic potential of cancer cells. In fact, the use of its inhibitors in pancreatic cancer appears to reduce the likelihood of metastasis [24,25]. In metastatic disease the IHH pathway is active not only in the tumor itself but also in the matrix microenvironment. The pathogenic factors that are mainly responsible for metastasis are not yet known, although there are several indications so far, eg snail, TGFβ, wnt, HGF [24,26,27].
The expression of the IHI index in archival material from the Laiko Hospital of Athens in 53 patients with diagnosed colorectal adenocarcinoma was studied by immunohistochemistry. This was followed by correlation with the age and sex of the patients, as well as with the location of the primary tumor in the large intestine and the presence or absence of distant metastases.
Statistical analysis
The Mann-Whitney U statistical test checks the equality of two means between independent samples. As a non-parametric test, it checks the correlation in populations in which normalcy cannot be ensured. The location of the cancer was defined as an independent variable. As the number of cancer cases located in the cation of the colon was small, two groups were formed, the first with the location of cancer in the orthosigmoid and the second with the location of the colon (anion or cation). The expression of the ihh index was the dependent variable in each case. 4 expression levels of each indicator were generated, depending on the signal strength of the cancer cells. The Spearmann Rho test was used to correlate age and IHH expression. Fisher’s exact test is used to look for correlations between categorical variables when the number of observations is small (<1000). It was used to find the statistical significance between the expression of the IHH index and the presence of distant metastases.
The location of the primary tumor is not related to the increased or not, expression of the marker, therefore any differences between in particular anion and cation colon (Table 1), due to embryogenesis do not affect the presence of these cancer stem cells. Age also does not show a statistically significant correlation, which means that younger ages are not characterized by higher rates of IHH (+) cells and similarly there does not seem to be a decrease over time (Figure 2). Next, sex is an independent factor that does not affect cancer stem cells. Finally, the expression of the index is statistically significantly related to the occurrence of distant metastases with p = 0.004. This indicates the most aggressive form of the disease in patients with IHH (+) stem cells (Figure 3).
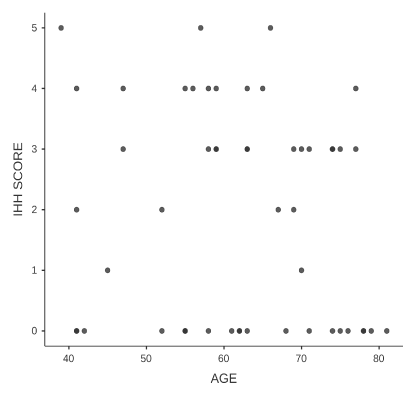
Figure 2. Age dispersion diagram in relation to the expression of ihh. In these diagrams the trend of the observations (dots) does not indicate any positive or negative correlation
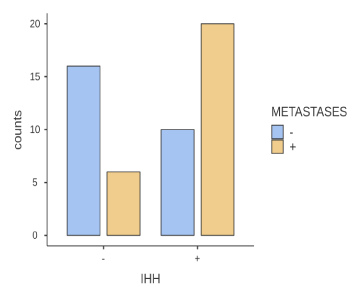
Figure 3. Histogram of presence of metastases and expression of ihh marker. The symbol (+) indicates the presence of metastases and the symbol (-) the absence of metastases. For pointers, the symbol (+) indicates an expression and (-) a non-expression. The vertical axis indicates the number of observations per case
Table 1. Frequencies of IHH score in the two major categories of colon
The activation of the IHH pathway in the gastrointestinal epithelium, but also in the other tissues results from the communication with factors of the layer. This is one of the key elements that changes in colon cancer where the pathway is activated independently of the factors of the matrix. When this happens, it causes the path to become overactive with negative course results. In fact, it changes its mode of operation, but also its target proteins, so the consequences cannot be predicted. Cells acquire new characteristics and avoid apoptotic signals.
The ligases expressed during IHH activation do not reach levels that suppress the pathway and this remains active, which may be justified by the disruption of communication with stromal cells. Inflammation of the large intestine promotes the development of sporadic cancer, so in studies in immunocompromised mice, the incidence of sporadic cancer was reduced. However, the use of IHH antagonists in inflammatory bowel disease has not been shown to protect against cancer.
The way the cancer pathway is activated, the differentiation of the relationship between cancer cells and the matrix, are the main points of the studies and the results are expected to help the medical community in the development of factors with good therapeutic results.
The results of our study are in line with the scientific knowledge so far, since the increased expression of the index is associated with the occurrence of metastases, making it necessary to find a therapeutic target.
None.
- Teglund S, Toftgård R (2010) Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim Biophys Acta 1805: 181–208. [Crossref]
- Varnat F, Duquet A, Malerba M, Zbinden M, Mas C, et al. (2009) Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Mol Med 1: 338-351. [Crossref]
- Büller NVJA, Rosekrans SL, Westerlund J, van den Brink GR (2012) Hedgehog signaling and maintenance of homeostasis in the intestinal epithelium. Physiology 27: 148–155. [Crossref]
- Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, et al. (2012) Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med 366: 2171-2179. [Crossref]
- Berlin J, Bendell JC, Hart LL, Firdaus I, Gore I, et al. (2013) A randomized phase II trial of vismodegib versus placebo with FOLFOX or FOLFIRI and bevacizumab in patients with previously untreated metastatic colorectal cancer. Clin Cancer Res 19: 258-267. [Crossref]
- Herter-Sprie GS, Kung AL, Wong KK (2013) New cast for a new era: preclinical cancer drug development revisited. J Clin Invest 123: 3639-3645. [Crossref]
- Jia Y, Wang Y, Xie J (2015) The Hedgehog pathway: role in cell differentiation, polarity and proliferation. Arch Toxicol 89: 179-191. [Crossref]
- Gerling M, Büller NVJA, Kirn LM, Joost S, Frings Z, et al. (2016) Stromal Hedgehog signalling is downregulated in colon cancer and its restoration restrains tumour growth. Nat Commun 7: 12321. [Crossref]
- Mazumdar T, DeVecchio J, Agyeman A, Shi T, Houghton JA (2011) The GLI genes as the molecular switch in disrupting Hedgehog signaling in colon cancer. Oncotarget 2: 638-645. [Crossref]
- Rodriguez-Blanco J, Schilling NS, Tokhunts R (2013) The hedgehog processing pathway is required for NSCLC growth and survival. Oncogene 32: 2335–2345.
- Pelczar P, Zibat A, van Dop WA, Heijmans J, Bleckmann A, et al. (2013) Inactivation of Patched 1 in mice leads to development of gastrointestinal stromal-like tumors that express Pdgfralpha but not kit. Gastroenterology 144: 134-144.e6. [Crossref]
- Tian H, Callahan CA, DuPree KJ, Darbonne WC, Ahn CP, et al. (2009) Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc Natl Acad Sci USA 106: 4254-4259. [Crossref]
- Mao J, Ligon KL, Rakhlin EY, Thayer SP, Bronson RT, et al. (2006) A novel somatic mouse model to survey tumorigenic potential applied to the Hedgehog pathway. Cancer Res 66: 10171-10178. [Crossref]
- Zhao C, Chen A, Jamieson CH, Fereshteh M, Abrahamsson A, et al. (2009) Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature 458: 776-779. [Crossref]
- Merchant M, Evangelista M, Luoh SM, Frantz GD, Chalasani S, et al. (2005) Loss of the serine/threonine kinase fused results in postnatal growth defects and lethality due to progressive hydrocephalus. Mol Cell Biol 25: 7054-7068. [Crossref]
- Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414: 105-111. [Crossref]
- Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, DMcIntyre D, et al. (2009) Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324: 1457-1461. [Crossref]
- Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, et al. (2014) Depletion of carcinoma-associated fibroblasts and fibrosis induces immune suppression and accelerates pancreatic cancer with reduced survival. Cancer Cell 25: 719-734. [Crossref]
- Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, et al. (2014) Stromal elements act to restrain, rather than support pancreatic ductual adenocarcinoma. Cancer Cell 25: 735-747. [Crossref]
- An Y, Cai B, Chen J, Lv N, Yao J, et al. (2013) MAP3K10 promotes the proliferation and decreases the sensitivity of pancreatic cancer cells to gemcitabine by upregulating Gli-1 and Gli-2. Cancer Lett 329: 228-235. [Crossref]
- Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo, Castillo-Martin M, Quinn SA, et al. (2012) Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumorinitiating cells. Cancer Cell 22: 373-388. [Crossref]
- Ramaswamy B, Lu Y, Teng KY, Nuovo G, Li X, et al. (2012) Hedgehog signaling is a novel therapeutic target in tamoxifen-resistant breast cancer aberrantly activated by PI3K/AKT pathway. Cancer Res 72: 5048-5059. [Crossref]
- Das S, Samant RS, Shevde LA (2013) Nonclassical activation of Hedgehog signaling enhances multidrug resistance and makes cancer cells refractory to smoothened-targeting Hedgehog inhibition. J Biol Chem 288: 11824-11833. [Crossref]
- Gu D, Liu H, Su GH, Zhang X, Chin-Sinex H, et al. (2013) Combining hedgehog signaling inhibition with focal irradiation on reduction of pancreatic cancer metastasis. Mol Cancer Ther 12: 1038-1048. [Crossref]
- Bailey JM, Mohr AM, Hollingsworth MA (2009) Sonic hedgehog paracrine signaling regulates metastasis and lymphangiogenesis in pancreatic cancer. Oncogene 28: 3513-3525. [Crossref]
- Inaguma S, Kasai K, Ikeda H (2010) GLI1 facilitates the migration and invasion of pancreatic cancer cells through MUC5AC-mediated attenuation of E-cadherin. Oncogene 30: 714-723 [Crossref]
- Joost S, Almada LL, Rohnalter V, Holz PS, Vrabel AM, et al. (2012) GLI1 inhibition promotes epithelial-to-mesenchymal transition in pancreatic cancer cells. Cancer Res 72: 88-99. [Crossref]



