The present study aims to investigate with functional MRI the neural correlates of imitative perspective-taking. A previous behavioural study on control subjects and callosotomized patients showed that, when subjects are free to reproduce intransitive gesture observed, imitation occurs mainly in mirror mode (60% in control subjects, 66% in patients, p<0.05); on the contrary, when asked to use the same or the opposite limb as the model, controls imitate in anatomical mode (93%, p<0.01), and patients in mirror mode (61%, p<0.05).
A subsequent fMRI study performed in control subjects described different cortical activation in the two conditions: left medial frontal gyrus (area 6), left inferior parietal lobule (area 40), opercular part of left inferior frontal gyrus (area 44), and bilateral parietal opercular cortices were activated in Imagine-to-Imitate condition only.
Functional MRI was here performed in 6 patients, 5 of whom participated also in previous behavioural study. Blood oxygenation level-dependent (BOLD) responses to gestures observation and imagery were analyzed.
Differences in BOLD signals between functional runs performed in Observe and Imagine to imitate conditions were observed, in particular respect to control healthy subjects. Specifically, at variance with previous results in control subjects, in Imagine-to-Imitate condition the cortical activation was inconsistent across patients. This observation is in line with behavioural results, demonstrating a diffuse inability of callosotomized patients to perform anatomical imitation.
Present data further suggest that the neural circuitry underpinning the anatomical imitation of intransitive gesture likely requires the cooperation of both hemispheres, and therefore the integrity of the corpus callosum.
Imitation, anatomical perspective, mirror-mode perspective, intransitive gestures, fMRI cortical activation, corpus callosum (CC), split-brain
Imitation is a behavioural event reproducing observed actions. In experimental conditions the actions to be imitated are usually presented when the imitator is facing the model [1], the typical position in which people interact in everyday life. When asked to imitate someone gesturing, an imitator can choose between two modes of imitation: a mirror (specular) way, i.e., using a right limb to copy a spatially matched left limb gesture of the facing model, or an anatomical mode, i.e., using a right limb for imitating an anatomically matched right limb gesture by the model.
Data from previous studies [2,3] setted in behavioral context demostrated that healthy subjects tend to prefer the mirror mode imitation when let free to imitate a model gesturing in 3rd person-perspective, and to imitate in anatomical mode when asked to perform with the same (or the opposite) limb respect the model’s [2]. At variance, callsotomized patients chosed the mirror mode imitation in both conditions [3]. Results led to hypothesize that the mirror mode of imitation would recruit the mirror neuron system, whereas the anatomical mode of imitation might be the expression of the mental rotation mechanism.
Subsequently, fMRI was performed in 10 control subjects of the previously tested groups, asked to Observe or Imagine-to-Imitate-with-the-same-limb, in separete runs, intransitive gestures [4]. Different cortical activation in the two conditions were observed: opercular part of left inferior frontal gyrus, left inferior parietal lobule, right temporo-parietal junction and bilateral parietal opercular cortices were activated in Imagine-to-Imitate condition only. These results confirmed previous behavioural observations and indicated that neural circuitry underpinning the anatomical imitation of intransitive gesture likely requires the cooperation of both hemispheres, and therefore the integrity of the corpus callosum [4].
To give firther support to this hypothesis, the same functional protocol was administerd to six callosotomized patients, who had undergone partial or total resection of the CC to treat drug-resistant epilepsy. Some of these patients took part to the behavioural study. Among the six patients participating in the present study, only patient P6 was able to perform anatomical imitation, as tested in behavioural protocol ([3], for patients P1-P5, present data for patient P6). Also in this case, a combination of observation and imagery was applied, to test the above-mentioned hypothesis, by analyzing differences in blood oxygen level dependent (BOLD) signals in the following conditions: 1. observing video clips depicting a model executing intransitive gestures (OBS run); 2. imagining to reproduce the gestures shown, using the same limb as the model (IIMsL run).
It resulted that the sole observation evoked less consistent activation of the cortical circuitry of the mirror neuron system (MNS) [5-7], and of the cortical areas involved in the planning the execution of the voluntary movements [8], than in the control subjects [4]. When asked to imagine to imitate using the same limb as the model (IIMsL), a different activation pattern was elicited from controls in this second tasks, in that bilateral activation of parietal opercula was observed only in two patients, one of whom did perform anatomical imitation.
Present results confirm that the CC, or at least part of it, is indispensable to imitate intransitive meaningful gestures with an anatomical perspective. Some of the results have been presented in abstract form [9,10].
Participants
The data were collected from 6 callosotomized patients (right-handed males) (Figure 1) (Table 1). The callosotomy was performed to treat drug-resistant epilepsy. All participants had normal or correct-to-normal visual acuity. Handedness was evaluated by the Oldfield inventory [11]. All patients gave their informed consent to participate in the study. The experimental protocol was approved by the Ethics Committee of Università Politecnica delle Marche, Ancona, Italy. Patients P1-P5 also participated in the previous behavioural study [3]. P6 was a new entry and was tested with the same neuropsychological protocol as other patients [3], just before the fMRI scan.
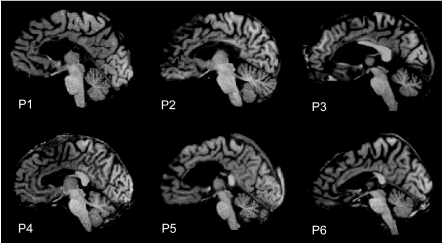
Figure 1. MR images of midsagittal brain slices from the 6 patients participating in the study: two of them (P1 and P2) underwent to total callosal resection, the others (P3-P6) to partial anterior callosal resection
Table 1. Summary data of callosotomized patients
Stimuli and tasks
The stimuli were 12-s video clips showing a model in third-person perspective performing two intransitive gestures (Figure 2). They were edited using E-PRIME software (Psychology Software Tools Inc., Pittsburgh, PA), and were presented during the fMRI session according to a block-designed protocol alternatining 12-s periods of rest and stimulation.

Figure 2. Functional MRI design. The functional design consisted of 2 identical runs composed of 13 resting periods alternating with 12 stimulation periods. The stimuli were presented in the following order: 1. silence gesture executed by the model with her right limb (SR; A); 2. bye-bye gesture executed by the model with her left limb (BL; B); 3. silence gesture executed by the model with her left limb (SL; C); 4. bye-bye gesture executed by the model with her right limb (BR; D). During each stimulation block each image was flashed 4 times for 3 s. Blocks were not randomized. From [4]
The video clips were among the stimuli used in the previous behavioural study [2], depicting a model executing intransitive gestures with her upper limbs: one of the two gestures was a body-related gesture (silence gesture: closed hand with index finger upright, close to the lips; (Figure 2), and the other was a body-unrelated gesture (bye-bye gesture: open hand, waving 45° to one side and the other, repeatedly (Figure 2). Each gesture was performed with either limb, in separate stimulation periods.
Each 5-min functional run started with a 12-s rest period (baseline), in which a fixation cross was presented in the center of a grey background, followed by the first task period. In each 12-s task period, a video clip depicting the same gesture was flashed 4 times. The same alternation of rest and stimulation periods was administered for all the four video clips.
At the beginning of the video clip both model’s arms were relaxed along the body, then the gesture was executed by the model who then returned to the initial standing position.
The following two conditions were investigated:
- Observation (OBS): the subject was required to carefully observe the video clips. The instruction was: "Please, simply look at the video".
- Imitative imagery (IMsL): the subject observed the videos and was requested to imagine himself performing the movement with the same limb used by the model. The instruction was "Please, as soon as you see the gesture of the model imagine to reproduce the same gesture with the same limb used by the model".
Functional MRI stimulation protocol
Before the scanning sessions, subjects received verbal information about the experiment. The fMRI sessions consisted of 2 functional runs, each containing 13 resting periods alternating with 12 stimulation periods, lasting 12 s each. Functional run started and ended with a resting period; during each stimulation period a single gesture was flashed 4 times, each time for 3 s (Figure 2). The stimulation blocks were presented within the run in the following sequence: Silence Right (SR; Figure 2), Bye-bye Left (BL; Figure 2), Silence Left (SL; Figure 2) and Bye-bye Right (BR; Figure 2).
The videos were presented through VisualStim Digital glasses (Resonance Technology, Inc.) that each participant wore before entering the scanner. To reduce head motion artifacts during the data acquisition, we used a custom-head support.
After the scanning session, the patients were asked which limb they imagined to use to imitate the model’s gesture in the screen: all patients answered they imagined to use their right limb when the model used “her limb on the right”, and viceversa; their statement seem to indicate they adopted an anatomical strategy of imitation. However, we had good reasons to suspect that the definition “her limb on the right “likely means “her hand in the right side of the screen” and therefore, since the model was facing the subjects (third person presentation, or 180°), the imitation strategy could have been specular rather than anatomical.
Functional MRI data acquisition
Data were collected using a 1.5 T (Signa Excite NV/i CV/i, General Electric Medical System, Milwaukee, WI, USA) equipped with 50 mT/m gradients. Images were then transferred to a Unix workstation (General Electric Advantage Windows 4.2) and finally to a computer.
Subjects, with their head restrained within a circularly polarized head coil, were invited to wear 3D glasses, to lie down in a supine position, avoiding even minimal movement.
Image acquisition occurred through 4 steps:
- acquisition of anatomical three-plane localizer (2D SPGR, TR 120 ms, TE 15 ms, Flip Angle 70°, FOV 23 × 23 cm, slice thickness 5 mm, Matrix 256 × 256, 1 Nex, scan time 31 s).
- acquisition of a 3D data set (IR Prep Fast SPGR; TR 15.2 ms, TE 6.9 ms, TI 500 ms, Flip Angle 15°, FOV 29 × 29 cm, slice thickness 1 mm, Matrix 288 × 288, 1 Nex, scan time 8:20 min).
- acquisition of 20 contiguous 5-mm-thick axial or oblique functional images with a single-shot T2*-weighted gradient-echo EPI sequence (TR 3000 ms, TE 60 ms, Flip Angle 90°, FOV 28 × 21 cm, Matrix 96 × 64, 1 Nex, scan time 5:12 min).
- high-resolution axial (or oblique) anatomical images acquired from 20 selected planes (2D SPGR, TR 100 ms, TE 12 ms, Flip Angle 70°, FOV 28 × 21 cm, thickness 5 mm, Matrix 256 × 256, 1 Nex, scan time 2:25 min for 20 images) to superimpose functional activation images onto the anatomical landmarks, allowing to show blood vessels considered as possible sources of BOLD signals.
Two thousand axial or oblique functional images (100 per section, 1 image/3 s) were acquired during the stimulation cycle from 20 contiguous 5-mm-thick axial sections obtained from 20 previously selected planes. Functional images were obtained with the BOLD method. The axial planes were orthogonal to both the sagittal and the coronal planes, and their orientation was parallel to the AC-PC line.
Functional MRI data analysis
BrainVoyager software package (BrainVoyagerQX, Version 2.3.1.1770, 32-bit, Copyright © 2001-2014 Rainer Goebel) was used for analyzing data (DICOM format) that were loaded and converted into BrainVoyager’s internal ".fmr" data format. Data were preprocessed and analyzed using BrainVoyager QX 2.1 (BVQX; Brain Innovation, Maastricht, The Netherlands). We performed corrections for slice scan time; each functional volume for a given participant was aligned to the functional volume collected closest in time to the anatomical volume. Functional data were superimposed on anatomical brain images, aligned on the AC–PC line, and transformed into Talairach [12] space and co-registered with the anatomical image for each participant. Talairach transformation was performed using standard BVQX procedures [13].
Intensity inhomogeneity correction (IIHC) BrainVoyager tool was applied and a standard sequence of preprocessing steps performed. As it is impossible to lie completely still during a time interval the entire scanning session, and since physiological noise as well as physical (scanner-related) noise can reduce substantially the power of statistical data analysis, in order to minimize the false positive activations while increasing sensitivity to true task-related activations, slice scan timing (sinc interpolation based on information about the TR = 3000 ms), 3D correction for motion artefacts and temporal filtering were applied. No low-pass temporal or spatial filters (i.e., as the False Discovery Rate approach used for the specification of an appropriate threshold of statistical maps avoided spatial smoothing) were used.
After creating a functional project from measured DICOM files, the original voxel data were stored in STC (slice time course) files.
For each patient, the functional images of each functional run were co-registered and aligned to the three-dimensional high-resolution images and finally transformed into Talairach space. The first two images of each functional series were discarded to take into account signal intensity variations due to progressive saturation.
In order to investigate BOLD signals relative to the two functional runs (OBS and IIMsL), single-subjects contrasts for each run were obtained as follows. First, to determine whether gestures to be observed (OBS) differed from the same to imagine to be imitated (IIMsL), a Geneal Linear Model (GLM) approach was used to generate statistical parametric maps. In single-subjects GLMs, the predictor time courses were convolved with a standard hemodynamic response function (HRF) to account for the hemodynamic delay. After computing statistical maps for each individual, the VTC files from multiple subjects were submitted to multi-subjects analyses where the statistical maps containing estimated effects (beta values) separately for each subject were the inputs. Two kinds of multi-subjects analysis was performed: one grouping all patients, and another grouping separately patients with total and those with partial callosotomy.
Activation foci were studied by selecting Regions-Of-Interest (ROIs) in frontal, parietal and temporal cortices.
Under the assumption that voxels (or vertices) with the same coordinates in different brains access corresponding brain regions, in order to integrate the data from multiple subjects into a single GLM analysis, i.e., to achieve better comparison across voxels and to normalize the variance of the individual runs, raw fMRI time course z-normalization was calculated. When the activation coincided with the stimulation pattern, it was assumed to be evoked by the specific predictor.
Contrasts were used to test for differences between the two experimental conditions: OBS and IIMsL.
This study aimed at testing the hypothesis that BOLD signals evoked in OBS and IIMsL runs evoke different patterns of cortical activation. Using a subtraction method, BOLD signals intensity differences (contrasts) between the two runs were observed. The activation threshod was kept very selective to be sure all activations observed were due to the specific task.
Separated analysis was performed for each of the following conditions (c):
c1 = All gestures together
c2 = Gestures executed by the model with her right limb
c3 = Gestures executed by the model with her left limb
The activations evoked by a single gesture (bye-bye or silence) performance were not reported since the BOLD signal was too low to be significant. As said in the Methods, two kinds of multi-subjects analysis was performed: one grouping all patients, and another grouping separately patients with total and those with partial callosotomy. However, the interindividual variability produced a lack of responses in multisubject analysis which, therefore, has not further considered.
Behavioural result of patient P6
Before the fMRI session, patient P6, who did not participated in the previous behavioural study, was administered the behavioural protocol [3] to assess his ability to perform anatomical imitation. He produced 20/24 responses (83%) with anatomical perspective during the free session, and 23/24 (96%) during the driven session; thus indicating he was able to perform intransitive gestures imitation with an anatomical perspective.
Brain areas activated during OBS task
In the frontal lobe, the activation of area 6 in the left or right hemisphere was observed in c1 in three patients; activated foci were present also in area 4 (P1), area 5 (P6) and 46 (P5). Similar patterns of activation were observed.
In the parietal lobe, activation was observed in precuneus (PrCu; Ba7) in both hemispheres in 3 patients, in the right hemisphere in 1, in c1; in the superior parietal lobule (SPL; area 7) the activation was bilateral in c1 in 1 patient only (Table 2). In c1 activation of right area 40 was observed in 2 patients. In c2, the right precuneus was activated in 2 patients, the left in 1; left SPL, right angular gyrus and right IPL were activated in each of three different patients. In c3, only the right precuneus was activated in patient P4.
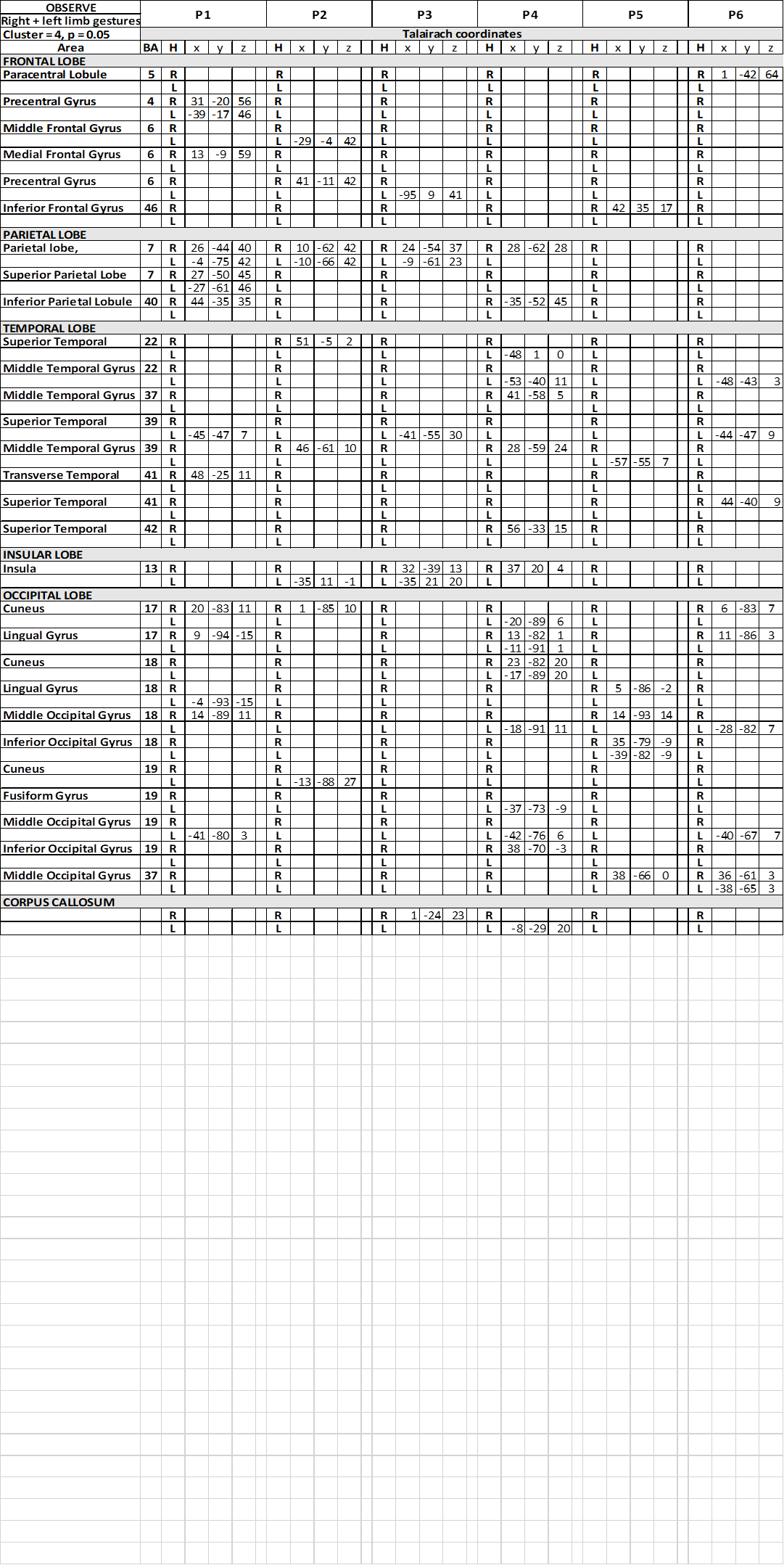
Table 2. Talairach coordinates of activation foci evoked in OBS condition in each patient
In the temporal lobe, in condition c1, the activation of area 22 in the left superior temporal gyrus (STG) was reported in 1 patient (Table 2), and in the right in 1 patient. The activation of left area 39 of STG or MTG was observed in 4 patients, and in the right hemisphere in the remaining 2. In c2 and c3 the activation was inconsistently distributed, in areas 22, 37, 39 and 41, either in the left or in the right hemisphere.
In all conditions, activation of visual areas of the occipital lobe has been observed in both hemispheres (summarized in Table 2 and Figure 3): activation pattern was not homogeneous through the patients, but in all of them a visual areas in each hemisphere was activated.
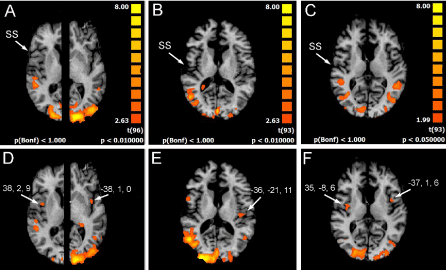
Figure 3. Significant activation in the different runs. (A), (B) (C), OBS condition in three patients (P1, P2, P6): the activation of visual areas in the occipital cortex of both hemispheres is visible. No activation is evident in the parietal opercula. (D) (E) (F), IIMsL condition in the same three patients: the activation of visual areas in the occipital cortex is still present. In D and F (P1 and P6) activation foci in the opercular cortex (arrows) of both hemispheres are visible, in E (P2) in left hemisphere only. In each condition, axial images are from the same z values for patients P2 and P6; in patient P1 the two hemispheres are shown at different z values because of different position of the opercular activation foci. SS, sylvian sulcus; according to the radiological convention, the left hemisphere is shown on the right
In the insular lobe, in condition c1, activation was observed in the right insula in one patient, in the left in another, and bilaterally in a third. In c2 activation was observed in the right insula in 3 patients, and in the left in 1; in c3 activation was observed in the left insula in 1 patient, and bilaterally in 2.
In patients P3 and P4, an activtion focus was observed in the spared portion of the CC.
Brain areas activated during IIMsL task
During IIM run, the contrast task IIM versus baseline resulted in map of active voxel clusters, whose Talairach coordinates are reported in Table 3.
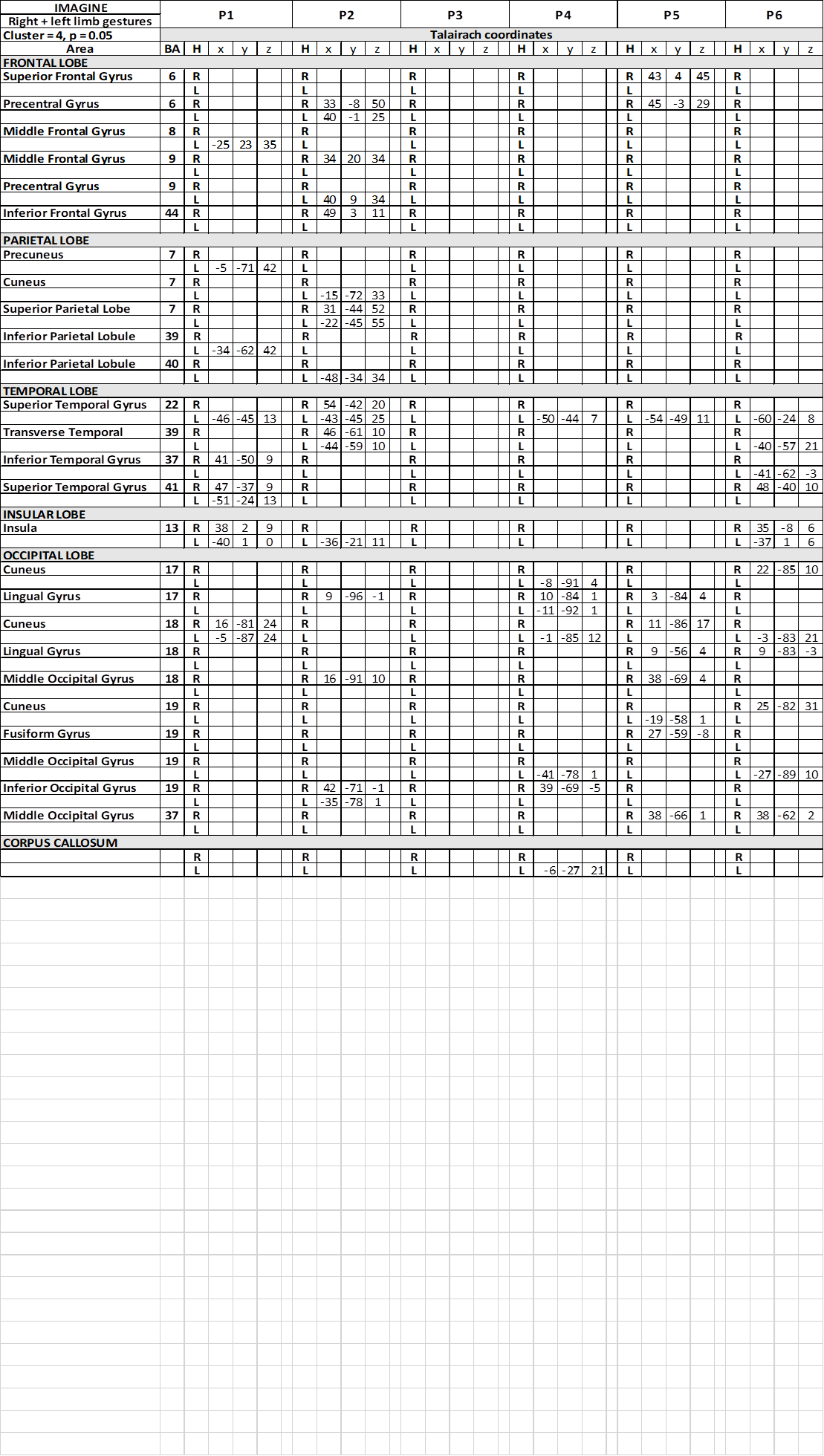
Table 3. Talairach coordinates of activation foci evoked in IIMsL condition in each patient
In condition c1, activation foci in the frontal lobe were observed in precental gyrus (area 6), bilaterally in patient P2 and in the right hemisphere in P5; in middle frontal gyrus (area 6) in 2 patients, in the right side in one patient, in the other in the left (Table 3); in 1 patients activation was observed in right IFG (area 44). In c2 and c3, a similar scattered activation was observed, although bilateral activation was not observed in any patients.
In the parietal lobe, activation was observed in conditions c1 in the left PrCu in 1 patient (area 7; Table 3); bilateral activation was observed in the superior parietal lobule (SPL; area 7) in patient P2, who also displayed activation in left IPL (area 40). In conditions c2 and c3, activation was observed in different patiens in PrC, SPL and IPL, never bilateral.
In the temporal lobe the activation of area 22 in the STG was also reported in c1, bilateral in 1 patient, in the left hemisphere in 4 (Table 3). Bilateral activation of area 39 was also observed in the same patient, and of area 41 in another. In c2 and c3 activation was rather casual (Table 5) (Table 7).
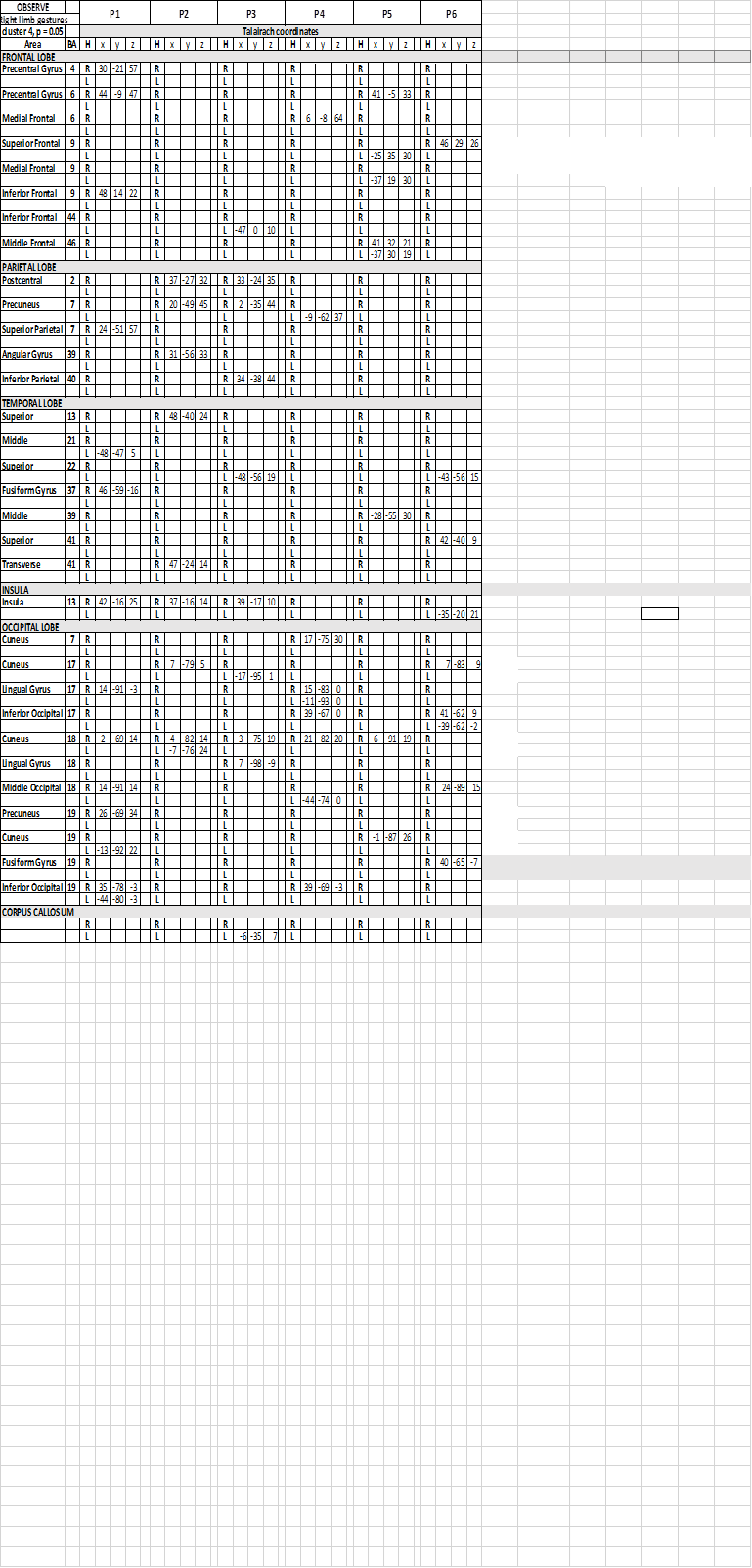
Table 4. Talairach coordinates of activation foci evoked in OBS condition in each patient, observing gestures performed by the model with her upper right limb
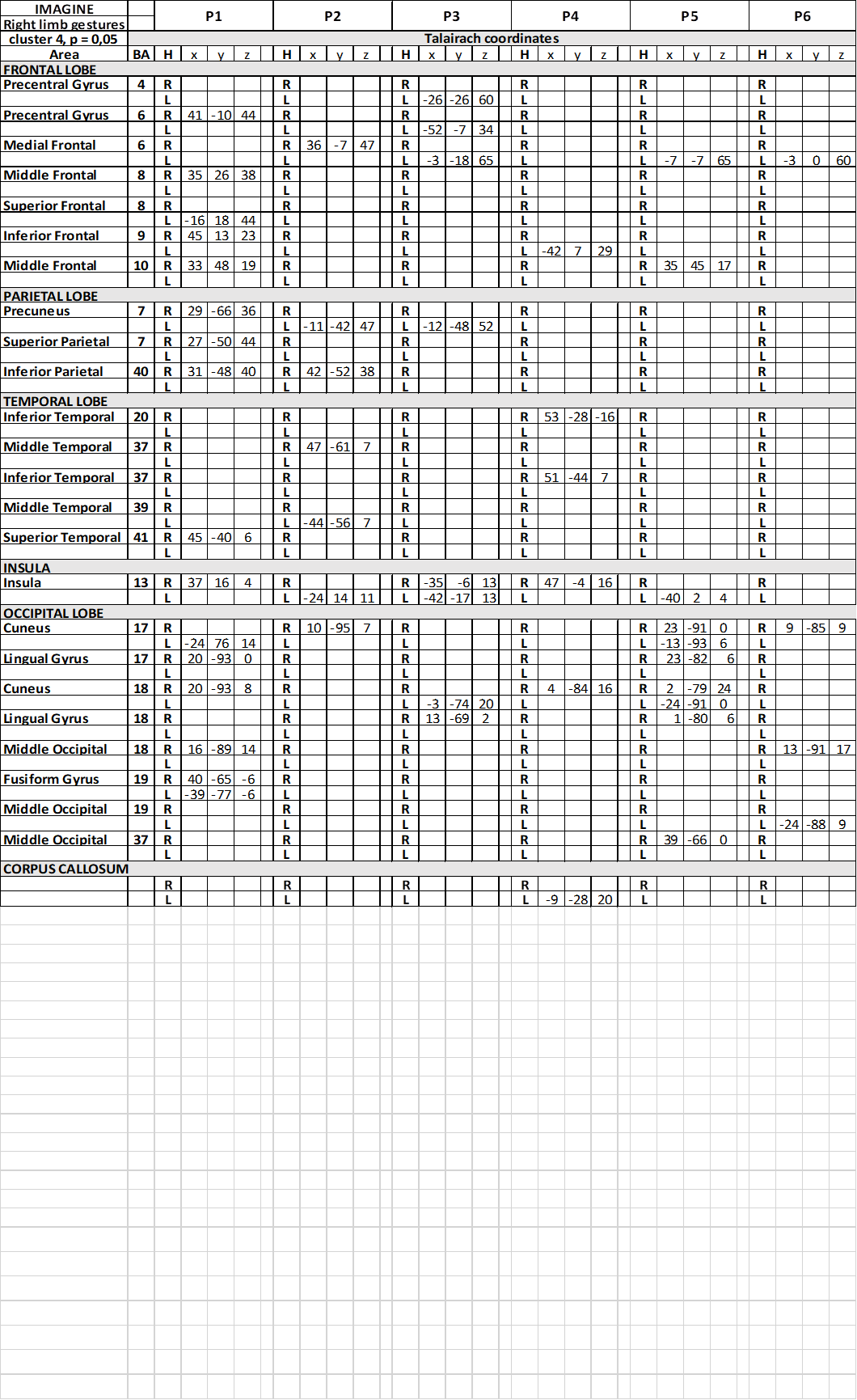
Table 5. Talairach coordinates of activation foci evoked in IIMsL condition in each patient, imaging to imitate gestures performed by the model with her upper right limb
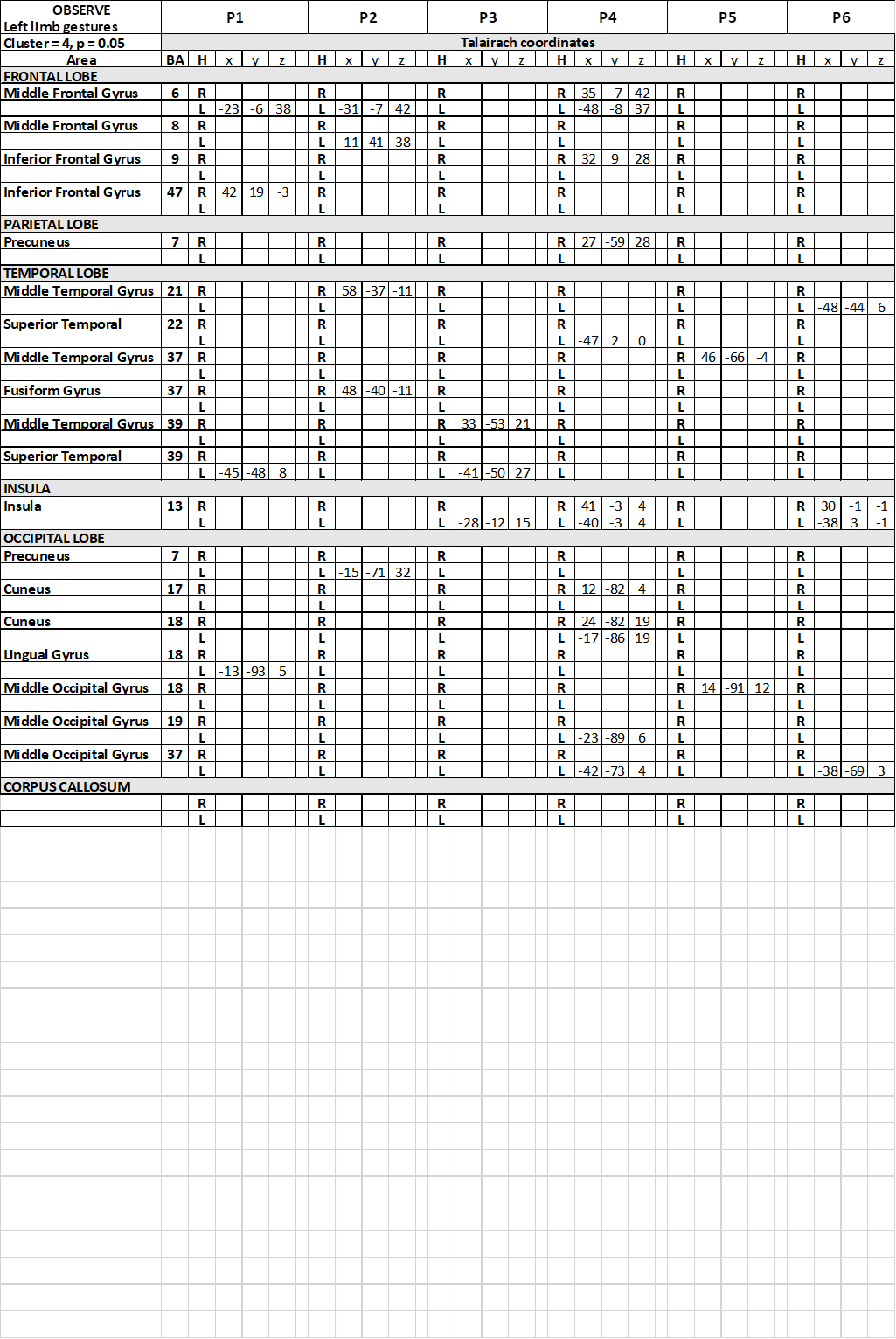
Table 6. Talairach coordinates of activation foci evoked in OBS condition in each patient, observing gestures performed by the model with her upper left limb
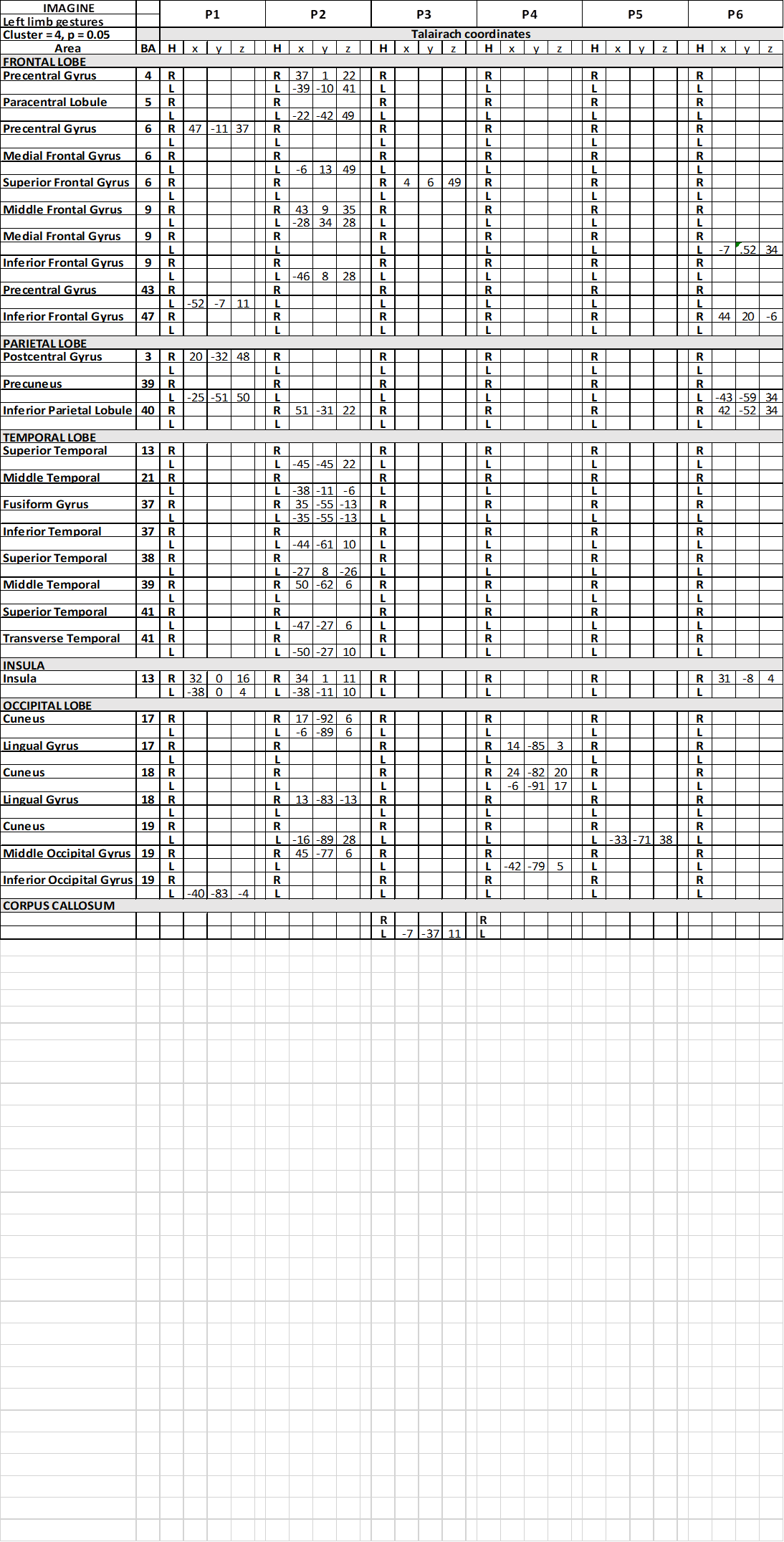
Table 7. Talairach coordinates of activation foci evoked in IIMsL condition in each patient, imaging to imitate gestures performed by the model with her upper left limb
In all conditions, in the occipital lobe the activation of visual areas has been observed in both hemispheres (Tables 3) (Table 5) (Table 7) (Figure 3), inconsistent in different patients.
The most interesting observation was the activation of the middle insula (area 13) in both hemispheres in c1 in patients P1 and P6 (Figure 3) (Table 3). In conditions c2 and c3 the activation of parietal operculum was mainly in the left hemisphere (c2) (Table 5), or in both hemispheres in 2 patients and in the right in 1 (c3) (Table 7).
Activation was also observed within the spared portion of corpus callosum, in the middle portion of the body, in patient P4 in c1 and c2 (Table 3) (Table 5), and in P3 in c7 (Table 7).
The present research was designed to countercheck the hypothesis that the neural circuits underpinning the anatomical imitation consists in a distributed network involving both hemispheres, and to demonstrate the role of the CC. To this purpose, in the present study the same fMRI protocol previously presented to healthy control subjects [4] was administer to 6 out of the callosotomized patients previously tested with behavioural protocol [3]. The results indicated that in callosotomized patients the same cortical areas as the control subjects are activated in the two conditions (OBS and IIMsL), although less consistently than in control subjects. This observation points to a crucial role for the CC, the lack of which is the main difference between patients and controls.
Present research follows previous behavioral studies investigating the strategies used by healthy subjects (controls) and callosotomized patients in imitating intransitive gestures [2,3]. Behavioral results demonstrated that: 1. the mirror mode of imitation was preferred in free sessions both from control subjects (61%) and patients (66%); the anatomical perspective is privileged in driven sessions by controls (93%), but not by patients, who still preferred specular modality (61%); 2. in driven sessions, the terms same and opposite were interpreted according to an anatomical perspective by control subjects, likely in a specular perspective in patients. In addition, hand preference did not correlate with action observation and execution (imitative act; see [2,3]).
In a subsequent imaging study [4], BOLD fMRI was used in healthy control subjects to identify the cortical activations evoked by observing intransitive gestures performed by a model in video clips and those evoked by imaging to imitate the same gestures with the same limb of the model. The results showed that the sole observation of intransitive gestures activated the cortical circuitry of the MNS, and the cortical areas involved in the planning the execution of the voluntary movements. The imagery imitation of the gestures with the same limb as the model evoked a broader and greater activation pattern, involving cortical areas of both hemispheres; namely, during this second task, areas belonging to the MNS were recruited, together with areas probably afferent to the Theory of Mind (such as TPJ), and areas involved in the mental rotation mechanism [4].
It was thus confirmed that the neural network underpinning the anatomical imitation is distributed in both hemispheres; in addition, further support to the role of PO was provided, and the involvement of the CC was confirmed.
Stimuli and instruction variables.
The visuo-motor stimuli used in the present functional study were two meaningful gestures, one body-related (the silence gesture) and one body-unrelated (the bye-bye gesture), performed by the model with her right or left upper limb, as in the previous studies [2-4]. These two meaningful gestures have been selected since no difference in performance was previously observed between meaningful and meaningless gestures; in addition, the silence and bye-bye gestures are well known and easier to be executed, also with the imagination.
As explained in the previous paper [4], being the present work performed within the magnet, the stimulation protocol required some adaptations from the behavioral contest, since during a fMRI run it is necessary to avoid any movement. Therefore, the OBS and IIMsL modality have been used, and it was assumed that the OBS modality could be similar to the free imitation (occurring in a mirror mode, also in healthy subjects; [2,3]), and that driven imitation could be substitute in the magnet by an imagined imitation with the same limb as the model, in that from our previous paper it was evident that healthy subjects performed driven imitation in anatomical mode [2].
Observe run (OBS)
In this conditions, consistent activation, although not homogeneous across patients, was observed in the visual areas of both occipital lobes, as expected, since the visual stimuli were presented centrally in the visual field. In addition, the following areas were activated: the left medial area 6 of MFG in some patients and left IPL (area 40), likely belonging to the MNS. Activation was also frequently observed bilaterally in the SPL (area 7), and in some cases in the left temporo-parietal junction (TPJ; posterior area 22), likely related to the recognition of self/others body parts and movements. Activation was also observed in parietal operculum (PO) in some patients.
The present findings are in agreement with previous observations, reporting that the network of motor areas involved in preparation and execution of action was also activated by simple observation of actions [8] (see Discussion in [4]). In addition, present results confirm that also in split brain patients, during the OBS run, the recruitment of regions belonging to the MNS occurs, although less consistently than in healthy subjects, with the role to recognize the action [14,15]. As previously noticed, the fMRI is not able to detect the temporal succession of the activation, therefore it is not possible to define the order of activation, if any.
Image to imitate with the same limb runs (IIMsL)
In this condition, other than the above-mentioned pattern, activation was observed in the left TPJ including also area 39 (=angular gyrus, involved in the right-left recognition; [16]) more often than in OBS condition. Moreover, activation was observed in the insula of the PO (three patients) and in the area 44 in one patient.
As previously described (see Results and Discussion in [4]), to image imitating a gesture with the same limb of a model, a subject first have to observe the gesture. This step will activate mirror neuron system, to recognize the action and the body region used to perform the gesture [17]. All these activations could be the same as observed during the OBS task. In addition, to use the same limb, the subject will have first to recognize whether the model used her arm or leg, and whether her right or left limb, to execute the gesture. In these operations, the TPJ and IPL will be likely activated, dealing whit the recognition of the body part and with the shift from self to other [18]. Later, the subject will have to decide what the term “same” means: control subjects interpreted this instruction according to an anatomical criterion, as also demonstrated previously in a behavioural context [2]. Callosotomized patients, on the contrary, seem to adopt, also in this case, a spatial compatibility criterion [3], although they declared, when asked, to have used an anatomical one. Finally, the motor program for the imaged movement, according to the selected limb and anatomical/specular criterion, should be activated: this could be the role of the PO.
In the present study, the activation of left TPJ has been reported in all patients except one, who did not perform anatomical imitation. The activation of IPL was observed in only two patients, none of whom performing anatomical imitation. The activation of PO was observed in three patients, one of whom able to perform anatomical imitation (P6). These results once again indicate that a similar activation pattern is evoked in patients; however, because of the lack of the CC, the neural circuit is not completed, therefore it is impossible for the patients to perform anatomical imitation. Actually, the sole patient performing anatomical imtation, P6, shows some fibres spared in the central callosal body; other patients seem to have spared fibres in that region, P3 and P4 and perhaps P5; these three patients, however, did not perform the anatomical imitation. Some explanations could be suggested: a different level of attention or of instruction understanding during the task; an interindividual variation of CC morphology and/or fine topography; the fact that surgery may have altered the normal morphology of this brain region, and therefore compromised its function; an interindividual difference in the neural circuit recruited. Further studies would be necessary to clarify this point.
Neural mechanism: comparison with other studies
By analyzing the results of the present research also in the light of previous functional study carried out in similar condition, the model hypothesized in previous fMRI study in healthy subjects [4] can be proposed again, as follows: during the anatomical imitation of intransitive upper limb gesures, the following brain areas are specifically activated: area 6 of MFG, area 40 of IPL, and area 44 of IFG, all in the left hemisphere; posterior area 22 of TPJ in the right, and PO in the insula of both hemispheres. These activation foci were observed also in callosotomized patients, at least in some of them, although most did not perform anatomical imitation. As previously described, the activated areas could assume the following roles:
- the posterior SMA (area 6) would represent the spatial trajectory of the gesture to be imitated, independently from the body part executing that gesture [19]. Accordingly, brain damaged patients showed deficits in anatomical imitation, especially when lesion involved the left dorsal premotor cortex [20].
- the left area 44 would recognize and understand the observed action and transfer the information to the TPJ [21]. The activation of area 44 has been observed in previous finger and hand posture imitation studies [21-23].
- the TPJ would allow to shift from other/self gesture recognition [24], TPJ has been often observed to be involved in gesture imitative behaviour (see [25] for a review), as well as IPL for hand gesture imitation [26], mainly of meaningful gestures [27], in the left hemisphere [28]. The activation of TPJ has been previously described during imitative tasks, possibly involved in the control of the imitation in case of spatial incompatibility [18].
- the bilateral activation of the PO during the anatomical imitation of finger position was previously reported in healthy subjects [29], suggesting their implication in imitation tasks, particularly in coding the body part executing an action, rather than the action itself [29] (see below). A role for the PO in the anatomical imitation has been confirmed, either in the right hemisphere [30] or in both [31] (see also Discussion in [4]). Recently, the study of Tessari and coworkers [23] seems to indicate an involvement of PO in the imitation of intransitive meaningful gestures; such is observation likely suggests that this cortical area might be one step of the “semantic route”, likely used for the imitation of meaningful gestures [23,32].
Among the many papers published dealing with the imitation issue, in healthy adult and children, in patients with brain lesions, psychiatric alterations and also in callosotomized patients, the studies more similar to ours, for protocol uses and/or subjects studied, are those by Goldenberg and coworkers [33], by Lausberg and Cruz [34] and by Mengotti et al. [35]. The first paper reports a case of a patient whose CC was severed by an hemorrhagic lesion affecting the truncus and the splenium and causing somatosensory and visual disconnection of the hemispheres. In experimental conditions similar to ours, different hand postures respect to the head were presented in not lateralized way. The patient perfectly imitate the hand postures with both hands, according to an “obliged” mirror perspective. However, at variance with ours, Goldenberg’s patient received a clear instruction on the way to imitate, i.e., he should use his right or left hand in different sessions; our patient had to interpret the instruction (“use the same limb as the model” or “use the opposite limb of the model”), and this could account for the difference in the result between the two cases.
Lausberg and coworkers [34] examined three patients with complete callosotomy, four patients with partial callosotomy and 10 healthy subjects, imitating hand–head positions and finger configurations with non-lateralised and lateralized tachistoscopic stimulus presentation. One split-brain patient had severe right hemispheric deficit in imitating hand–head positions with her left hand, while finger configuration imitation was preserved. The other two split-brain patients had no impairment in hand–head position imitation. Also, these results are in agreement with ours, when considering the gesture imitation with the right or left hand, i.e., when the patients are not requested to interpret the instruction [3].
A neuroimaging study [36] related the imitation performances of meaningful and meaningless transitive and intransitive gestures with gray ad white matter lesions. It has been shown that a lesion in left superior parietal cortex, extending anteriorly to SMA, impairs all gestures imitative performance. However, not conclusive evidence were obtained for meaningful intransitive gestures, as those used in the present study are.
Finally, Mengotti and coworkers [35] invited patients with unilateral left or right brain damage to perform an ideomotor task and a gesture imitation task. The results show that lesions in the left or right hemispheres gave rise to different deficits: lesions in the left hemisphere impaired imitation when anatomical matching was required, and lesions in the right hemisphere impaired imitation when spatial matching was required. However, by carefully inspecting the data, the difference due to the hemispheric lesions are in relation to controls’ performance, and in fact the two patients’ groups, left- (LBD) and right-brain-damaged (RBD), perform with overlapping results, as also Authors state. In addition, both groups’ performance are worst in anatomical gestures imitation than in the mirror. These results are in agreement with ours and indicate that the anatomical imitation is more demanding the than mirror one in terms of interhemispheric cooperation.
A very recent review analyzing the neural correlates of the imitation of intransitive gestures [37] confirmed that “imitation is a complex function sustained by a network of bilateral brain areas”, in which the left hemisphere, more particularly the parietal cortex, has a key role in imitation of intransitive gestures. Similar conclusions were achieved in the recent paper by Tessari and coworkers [23], who analyzed the performances of left brain damaged patients when imitating meaningful and meaningless intransitive gestures. They showed that brain damaged patients performed worst than control subjects, and left-damage patients worst than right-damaged. In addition, finger were imitated worstly than hand meaningless gestures, while for meaningful gestures the performance was similar. However, these Authors did not pay attention to the imitation perspective and did not analyze the eventual perfomance’s difference when imitating with anatomical or mirror mode.
Present results seem to confirm that the imitation according to an anatomical criterion seems to require the cooperation of both hemispheres: the TPJ of the right, the MGF, IPL and IFL of the left, and the PO of both. Dorsal premotor area 6 of MGF communicate with the other hemisphere by sending fibers in the central portion of the CC [38]. Area 44 of IFG sends interhemispheric fibres through the ventral rostral body and ventral anterior midbody [38]. Callosal fibres arising from IPL (areas 39-40) cross the dorsal splenium, and those from TPJ (posterior area 22 and 39) cross the ventral splenium [38]. Concerning the trajectory of interhemispheric fibres connecting the opercular cortices of the two sides, previous studies seem to indicate the anterior callosal body for fibres connecting fronto-parietal opercula (gustatory areas, [39]), and central callosal body for fibres connecting parietal opercula (second somatosensory areas, [40-44]). Actually, an activation focus in the central CC body was observed in our previous paper on healthy subjects [4].
The involvement of the CC has been already suggested in a previous study reporting in children a gradual shift towards the anatomical imitation mode, as the maturation of the CC progress with age, from 8 to 18 years [45].
In conclusion, present results confirm that the lack of callosal fibres, particularly from anterior and/or central body, could impair anatomical imitation performance, although the involved cortical areas could be still activated. This observation is in line with previous studies, evidencing that imitation is sustained by a bilateral network [37,46], and suggesting that the distruption of intherhemispheric fibres, other than focal brain lesions, may impair anatomical imitation [37]. The resection of the callosal fibres, interrupting the neural circuit underpinning the anatomical imitation, makes impossible for cortical areas in both hemispheres to communicate and cooperate.
The Authors are especially grateful to Prof. Giovanni Berlucchi for initial stimulating suggestions, to all patients for participating in the study, to Gabriella Venanzi fo scheduling the patients’ exams, and to the radiology techncians Luigi Imperiale and Lucio Montesi for their invaluable help in collecting data during and soon after the volunteers’ fMRI sessions. Thanks are also due to Michele Ombrosi, for his contribution to the initial processing of fMRI data.
This work was supported by Ministero Istruzione, Università e Ricerca, PRIN 2009, and by Università Politecnica delle Marche, RSA 2010, RSA 2011.
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
- Chaminade T, Meltzoff AN, Decety J (2005) An fMRI study of imitation: action representation and body schema. Neuropsychologia 43: 115-127. [Crossref]
- Pierpaoli C, Ferrante L, Berlucchi G, Manzoni T, Fabri M (2014) Anatomical or mirror - mode imitation? A behavioral approach. Arch Ital Biol 152: 20-31.
- Pierpaoli C, Foschi N, Cagnetti C, Ferrante L, Manzoni T, et al. (2018) Imitation strategies in callosotomized patients. Arch Ital Biol 156: 12-26.
- Pierpaoli C, Fabri M, Polonara G (2020) Cortical activation during imitative behavior: an fMRI study. J Syst Integr Neurosci 7: 1-12.
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC (1999) Cortical mechanisms ofh uman imitation. Science 286: 2526-2528.
- Rizzolatti G, Fogassi L, Gallese V (2001) Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci 2: 661-670.
- Rizzolatti G, Craighero L (2004) The Mirror-Neuron System. Annu Rev Neurosci 27: 169-192.
- Calvo-Merino B, Glaser DE, Grèzes J, Passingham RE, Haggard P (2005) Action observation and acquired motor skills: an fMRI study with expert dancers. Cer Cor 15: 1243-1249.
- Pierpaoli C, Berlucchi G, Paggi A, Ferrante L, Manzoni T (2010) Anatomical and mirror imitation strategy in callosotomized patients. National Congress of Società Italiana di Fisiologia, Varese 15-17.
- Pierpaoli C, Berlucchi G, Paggi A, Manzoni T, Fabri M (2010) A behavioral study of gesture imitation: an anatomical or mirror-image strategy? 7th FENS Forum of European Neuroscience Societies, Amsterdam, July 3-7.
- Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 9: 97-113.
- Talairach J, Turnoux P (2008) Co-Planar Stereotaxic Atlas of the Human Brain. New York (USA): Thieme Medical Publishers.
- Goebel R Brainvoyager (1996) A program for analyzing and visualizing functional and structural magnetic resonance data sets. Neuroimage 3: 604.
- Rizzolatti G, Cattaneo L, Fabbri-Destro M, Rozzi S (2014) Cortical mechanisms underlying the organization of goal-directed actions and mirror neuron-based action understanding. Physiol Rev 94: 655-706.
- Rizzolatti G, Sinigaglia C (2016) The mirror mechanism: a basic principle of brain function. Nat Rev Neurosci 17: 757-765.
- Seghier ML (2013) The angular gyrus: multiple functions and multiple dubdivisions. The Neuroscientist 19: 43-61.
- Carmo JC, Rumiati RI, Vallesi A (2012) Understanding and imitating unfamiliar actions: distinct underlying mechanisms. PLoS ONE 7: e46939.
- Swoden S, Catmur C (2015) The role of the right temporoparietal junction in the control of imitation. Cer Cor 25: 1107-1113.
- Wong AL, Jax SA, Smith LL, Buxbaum LJ, Krakauer JW (2019) Movement imitation via an abstract trajectory representation in dorsal premotor cortex. J Neurosci 39: 3320-3331.
- Chiavarino C, Apperly IA, Humphreys GW (2007) Exploring the functional and antomical bases of mirror-image and anatomical imitation: the role of the frontal lobes. Neuropsychologia 45: 784-795.
- Molnar-Szakacs I, Iacoboni M, Koski L, Mazziotta JC (2005) Functional segregation within pars opercularis of the inferior frontal gyrus: evidence from fMRI studies of imitation and action observation. Cer Cor15: 986-994.
- Aziz-Zadeh L, Koski L, Zaidel E, Mazziotta J, Iacoboni M (2006) Lateralization of the human mirror neuron system. J Neurosci 26: 2964-2970. [Crossref]
- Tessari A, Mengotti P, Faccioli L, Tuozzi G, Boscarato S (2021) Effect of body-part specificity and meaning in gesture imitation in left hemisphere stroke patients. Neuropsychologia, 151: 107720.
- Jackson PL (2006) Neural circuits involved in imitation and perspective-taking. NeuroImage 31: 429-439.
- Berlucchi G, Vallar G (2018) The history of the neurophysiology and neurology of the parietal lobe. In: Handbook of Clinical Neurology, Vol 151, Chapter 1, Elsevier 3-30.
- Goldenberg G, Karnath HO (2006) The neural basis of imitation is body part specific. J Neurosci 26: 6282-6287.
- Lui F, Buccino G, Duzzi D, Benuzzi F, Crisi G, et al. (2008) Neural substrates for observing and imagining non-object-directed actions. Soc Neurosci 3: 261-275.
- Muhlau M, Hermsdörfer J, Goldenberg G, Wohlschläger AM, Castrop F (2005) Left inferior parietal dominance in gesture imitation: an fMRI study. Neuropsychologia 43: 1086-1098.
- Mengotti P, Corradi-Dell’Acqua C, Rumiati RI (2012) Imitation component in the human brain: an fMRI study. Neuroimage 59: 1622-1630.
- Kubiak A, Króliczak G (2016) Left extrastriate body area is sensitive to the meaning of symbolic gesture: evidence from fMRI repetition suppression. Sci Rep 6: 31064.
- Mengotti P, Ticini LF, Waszak F, Schütz-Bosbach S, Rumiati RI (2013) Imitating others’ actions: transcraniam magnetic stimulation on the parietal opercula reveals the processes underlying automatic imitation. Eur J Neurosci 37: 316-322.
- Tessari A, Canessa N, Ukmar M, Rumiati RI (2007) Neuropsychological evidence for a strategic control of multiple routes in imitation. Brain 130: 1111-1126.
- Goldenberg G, Laimgruber K, Hermsdo J (2001) Imitation of gestures by disconnected hemispheres. Neuropsychologia 39: 1432-1443.
- Lausberg H, Cruz RF (2004) Hemispheric specialization for imitation of hand-head positions and finger configurations: a controlled study in patients with complete callosotomy. Neuropsychologia 42: 320-334.
- Mengotti P, Ripamonti E, Pesavento V, Rumiati RI (2015) Anatomical and spatial matching in imitation: Evidence from left and right brain-damaged patients. Neuropsychologia 79: 256-271.
- Bonivento C, Rothstein P, Humphreys G, Chechlacz M (2014) Neural correlates of transitive and intransitive action imitation: An investigation using voxel-based morphometry. NeuroImage: Clinical 6: 488-497.
- Lesourd M, Osiurak F, Baumard J, Bartolo A, Vanbellingen T (2018) Cerebral correlates of imitation of intransitive grestures: an integrative review of neuroimaging data and brain lesion studies. Neurosci Biobehav Rev 95: 44-60.
- Chao YP, Cho KH, Yeh CH (2009) Probabilistic topography of human corpus callosum using cytoarchitectural parcellation and high angular resolution diffusion imaging tractography. Hum Brain Mapp 30: 3172-3187. [Crossref]
- Mascioli G (2015) Functional MRI cortical activations from unilateral tactile-taste stimulations of the tongue. Physiol Behav 151: 221-229.
- Fabri M, Polonara G (1999) Role of the corpus callosum in the somatosensory activation of the ipsilateral cerebral cortex: an fMRI study of callosotomized patients. Eur J Neurosci 11: 3983-3994.
- Fabri M, Polonara G, Del Pesce M, Quattrini A, Salvolini U, et al. (2001) Posterior corpus callosum and interhemispheric transfer of somatosensory information: an fMRI and neuropsychological study of a partially callosotomized patient. J Cogn Neurosci 13: 1071-1079.
- Fabri M, Polonara G, Mascioli G, Paggi A, Salvolini U, et al. (2006) Contribution of the corpus callosum to bilateral representation of the trunk midline in the human brain: a fMRI study of callosotomized patients. Eur J Neurosci 23: 3139-3148.
- Fabri M, Polonara G, Mascioli G, Salvolini U, Manzoni T (2011) Topographical organization of human corpus callosum: an fMRI mapping study. Brain Res 1370: 99-111.
- Polonara G, Mascioli G, Foschi N, Salvolini U, Pierpaoli C, et al. (2014) Further evidence for the topography and connectivity of the corpus callosum: an FMRI study of patients with partial callosal resection. J Neuroimaging 25: 465-473.
- Wapner S, Cirillo L (1968) Imitation of a model’s hand movements: age changes in transposition of left-right relations. Child Dev 39: 887-894.
- Caspers S, Zilles K, Laird AR, Eickhoff SB (2010) ALE meta-analysis of action observation and imitation in the human brain. NeuroImage 50: 1148-1167. [Crossref]









