Embryo fragmentation, or extrusion of cell fragments, is a naturally occurring phenomenon that may be associated with impaired embryo development, failed implantation and decreased pregnancy rates. Previous studies have shown improvement in clinical outcomes after microsurgery to aspirate these cell fragments from embryos early during the embryo cleavage process. This case series of three patients with infertility showcases the successful application of the defragmentation technique in blastocyst stage embryos after in-vitro fertilization. The timing of defragmentation allows for better identification of those growth-arrested embryos that would benefit from “rescue” defragmentation. All three embryos demonstrated continued expansion and development after defragmentation, and resulted in clinical pregnancies.
defragmentation, in-vitro fertilization, blastocyst, microsurgery, infertility
Selection of the “best” embryo is a key step for single embryo transfer (ET) after in-vitro fertilization (IVF), which is the preferred technique to achieve a singleton pregnancy [1]. Embryologists select an embryo for transfer based on grading systems that assess the quality of the embryo, and predict the likelihood of successful implantation [2]. Grading systems, such as one reported by Gardner et al, are often based on three parameters: blastocoel expansion and hatching status, compactness and size of the inner cell mass, and the number and cohesiveness of trophectoderm cells, as well as state of the zona pellucida [2,3].
During development, the central blastula may extrude apoptotic blastomeres, commonly referred to as extruded cell fragments. These fragments can be observed from day 2 of embryogenesis, at the start of the cleavage process of zygotes. This is a natural phenomenon commonly observed in embryo development [4,5]. It is estimated that about 40% of embryos demonstrate fragmentation during the first cleavage in-vitro [6]. Increased fragmentation, however, has been shown to limit subsequent blastomere development, sometimes leading to arrest of embryo growth [4,7]. In one study, embryos with greater than 15% fragmentation produced only half the number of good quality blastocysts, compared to the control group [8]. Further, studies have shown that increased embryo fragmentation is associated with decreased implantation [3,9,10] and pregnancy rates [5,9,11].
Evidence suggests that fragment removal improves embryo development [4] as well as implantation potential [9,10]. In recent studies, the reproductive outcomes resulting from transfer of defragmented embryos were either similar to [10] or better than that of high grade (control) embryos [5]. These studies in the literature report on outcomes after defragmentation of cleavage-stage embryos [6]; specifically, day 2 [4,5] or day 3 [9,10] embryos. This case series reports on the reproductive outcomes after defragmentation of reproductive outcomes after defragmentation of blastocyst stage embryos after in-vitro fertilization, prior to loading embryos for embryo transfer. Timing the defragmentation procedure at this stage allowed us to identify the embryos that displayed growth arrest and could be successfully rescued with defragmentation.
Methods
Controlled ovarian hyperstimulation (COS) was performed with gonadotropins for 10-12 days. Follicular growth and diameter was monitored using serial transvaginal ultrasounds as well as serum estradiol levels. When a dominant follicle was present, final oocyte maturation was triggered using human chorionic gonadotropin (hCG). Transvaginal ultrasound guided oocyte retrieval was performed about 35 hours after hCG administration. Collected oocytes were either stripped of cumulus cells and subjected to intracytoplasmic sperm injection (ICSI), or were left unstripped and exposed to washed sperm for conventional insemination. Oocytes were assessed for fertilization approximately 18 hours after insemination.
After 5 days of culture, embryos were scored using the Gardner grading system. Select fragmented blastocysts displaying arrest of growth were chosen for defragmentation. Fragment removal was performed in a micromanipulation dish. The embryo was stabilized by a holding pipette, as seen in Figure 1, and a 1460 nm wave length infrared diode laser was used to create a small hole in the zona pellucida. The biopsy pipette was placed inside the zona pellucida and apoptotic cell fragments were gently aspirated using negative pressure. The embryo was then returned to the culture dish and incubator. Embryos were reassessed to determine viability for embryo transfer or cryopreservation.
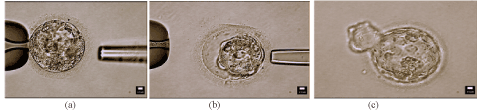
Figure 1. Photographs demonstrating an embryo before and after the defragmentation process, followed by successful development and subsequent embryo hatching. (a) Day 5 embryo before removal of extruded cell fragments; (b) Day 5 embryo after removal of extruded cell fragments; (c) Day 6 embryo “hatching” from zona pellucida
The following three cases demonstrate application of this aspiration technique and the reproductive outcomes associated with defragmentation of in-vitro fertilization blastocyst stage embryos. Clinical characteristics of the three patients are presented in Table 1.
Table 1. Patient clinical characteristics, results of oocyte retrieval, including number of oocytes undergoing normal fertilization (2PN) with either IVF or ICSI, number of embryos determined to be viable for cryopreservation, and whether pregnancy was achieved through subsequent embryo transfer
Case |
Couple age (years) |
Infertility diagnosis (duration in years) |
BMI (kg/m2) |
Labs |
Number of oocytes retrieved |
Number of oocytes fertilized via IVF, ICSI or cryopreserved |
Transferred versus implanted embryos |
Clinical Pregnancy |
1 |
26F/30M |
Anovulation, tubal factor (2) |
23.8 |
AMH 17.5 ng/mL
PRL 40.7 ng/mL
TSH 3.81 mU/L Free T4 5.5 ng/dl |
37 |
7 IVF
14 ICSI
7 cryopreserved |
1, 1 |
Yes |
2 |
36F/36M |
Tubal factor (5) |
23.2 |
AMH 8.8 ng/mL
TSH 2.13 mU/L
Free T4 14.9 ng/dl |
27 |
9 IVF
10 ICSI
7 cryopreserved |
1, 1 |
Yes |
3 |
42F/28M |
Diminished ovarian reserve (1) |
21.0 |
AMH 0.99 ng/mL
FSH 8.8 mIY/ml
PRL 19.0 ng/mL
TSH 5.070 mU/L
T4 5.2 ng/dl |
6 |
2 IVF
4 cryopreserved |
2, 1 |
Yes |
Case 1
The first case is a 26-year-old G2P0020 with a 2-year history of anovulatory infertility. She had undergone five ovulation induction cycles with clomiphene citrate prior to evaluation at the Reproductive Endocrinology and Infertility clinic. She underwent COS and oocyte retrieval. Due to this, embryo transfer was postponed. Most of the embryos were cryopreserved for future transfer. One of the embryos that demonstrated fragmentation was treated with fragment aspiration. A day after fragment aspiration, the blastocyst had hatched from the zona pellucida indicating continued blastocyst expansion. This defragmented embryo was later transferred, and the patient achieved clinical pregnancy .
Case 2
The second case is a 36-year-old nulliparous female with a 5-year history of infertility. After an abnormal hysterosalpingograhy, she underwent hysteroscopic polypectomy and laparoscopic bilateral salpingectomy for bilateral tubal occlusion. The patient underwent COS, hCG trigger and oocyte aspiration, in a procedure similar to that of Case 1. Preimplantation genetic testing for aneuploidy (PGT-A) was performed after the IVF/ICSI cycle. One of two cryopreserved euploid embryos was thawed, and found to have multiple fragments which were removed. As in Case 1, there was successful embryo hatching from the zona pellucida after aspiration of extruded cell fragments. The patient underwent transfer of the defragmented embryo, and successfully conceived.
Case 3
The last case is a 42-year-old G2P1011 with subclinical hypothyroidism who presented with infertility for one year. Evaluation confirmed diminished ovarian reserve. She underwent a similar procedure as in Cases 1 and 2, with COS, hCG trigger and oocyte aspiration. She declined PGT-A due to cost, and underwent fresh embryo transfer at the blastocyst stage in an IVF-ET cycle. This resulted in an early pregnancy loss. She then underwent a second IVF cycle with fresh embryo transfer with two defragmented blastocysts. She conceived and progressed to a term pregnancy.
Embryo fragmentation has been shown to be associated with stunted embryo growth, poor rates of implantation and unfavorable pregnancy outcomes. Previous studies have examined the effect of defragmentation on embryo viability, during the initial cleavage process on days 2 or 3. We present a case series in which three patients with differing infertility diagnoses underwent oocyte retrieval and IVF/ICSI. Of the embryos achieving normal fertilization, several were found to have apoptotic cell fragments present at the blastocyst stage. Those embryos that displayed growth arrest were subjected to defragmentation. Afterwards, evidence of continued blastocyst expansion was observed the following day. Defragmented embryos displayed appropriate progression, including successful “hatching” from the zona pellucida. To our knowledge, this is the first report in which extruded, apoptotic cells were aspirated in a clinical setting at the blastocyst stage, with evidence of continued blastocyst expansion, with evidence of continued blastocyst expansion. The timing of defragmentation allows embryos to progress beyond the physiologic defragmentation seen during cleavage, and if indicated by its morphological evaluation, to undergo a “rescue” defragmentation process with favorable pregnancy outcomes.
Several explanations for these findings have been proposed. Fragmentation is thought to be the result of programmed cell death, leading to the release of toxic substances that damage surrounding cells, or induce arrest of nearby blastomeres [4,9]. Further, the fragments may impede cell-to-cell communication by disrupting the spatial orientation of blastomeres or simply by crowding the space required for continued embryo cleavage or compaction. Some research suggests that fragments induce secondary degeneration in nearby structures. Blastomeres adjacent to extruded cell fragments have been found to have signs of vacuolar degeneration, seen on transmission electron microscopy [12,13]. With fragment removal, there is restoration of appropriate spatial relationship, and more adequate cell-to-cell communication [9,14]. The aspiration technique may also lead to a more accommodating microenvironment by removing toxic, apoptoic cell fragments [5].
In summary, this case series contributes to the literature by demonstrating that the defragmentation aspiration technique at the blastocyst stage may lead to improved blastocyst expansion, successful implantation and ultimately clinical pregnancy. The technique may represent a feasible option for improving clinical pregnancy and live birth rates in IVF/ICSI, particularly for patients with poorer quality embryos. Further comparative, prospective studies are necessary to evaluate the role of embryo defragmentation in improving pregnancy rates in IVF-ET cycles.
All authors made 1) substantial contributions to manuscript conception, design, and data analysis; 2) contributions in drafting the article or revising it critically; 3) final approval of the version to be published; 4) agreement to act as guarantor of the work.
The authors would like to thank Nicole Gilreath and Woojin Han for their assistance in data collection.
No funding to report.
No conflicts of interest to disclose.
- Practice Committee of the American Society for Reproductive Medicine (2017) Guidance on the limits to the number of embryos to transfer: a committee opinion. Fertil Steril 107: 901-903. [Crossref]
- Nasiri N, Eftekhari-Yazdi P (2013) An overview of the available methods for morphological scoring of pre-implantation embryos in in vitro fertilization. Cell J 16: 392-405. [Crossref]
- Gardner D, Lane M, Stevens J, Schlenker T, Schoolcraft W (2000) Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril 73: 1155-1158. [Crossref]
- Eftekhari-Yazdi P, Valojerdi MR, Ashtiani SK, Eslaminejad MB, Karimian L (2006) Effect of fragment removal on blastocyst formation and quality of human embryos. Reprod BioMed Online 13: 823-832.
- Kim SG, Kim YY, Park JY, Kwak SJ, Yoo CS, et al. (2018) Early fragment removal on in vitro fertilization day 2 significantly improves the subsequent development and clinical outcomes of fragmented human embryos. Clin Exp Reprod Med 45: 122-128. [Crossref]
- Halvaei I, Khalili MA, Safari S, Esfandiari N (2015) Ongoing pregnancies following cosmetic micromanipulation of preimplantation embryos in patients with implantation failure. Case Rep Med 734793.
- Keltz M, Fritz R, Gonzales E, Ozensoy S, Skorupski J, et al. (2010) Defragmentation of low grade day 3 embryos resulted in sustained reduction in fragmentation, but did not improve compaction or blastulation rates. Fertil Steril 94: 2406-2408.
- Alikani M, Calderon G, Tomkin G, Garrisi J, Kokot M, et al. (2000) Cleavage anomalies in early human embryos and survival after prolonged culture in-vitro. Human Reprod 15: 2634-2643. [Crossref]
- Alikani M, Cohen J, Tomkin G, Garrisi GJ, Mack C, et al. (1999) Human embryo fragmentation in vitro and its implications for pregnancy and implantation. Fertil Steril 71: 836-842.
- Keltz M, Skorupski JC, Bradley K, Stein D (2006) Predictors of embryo fragmentation and outcome after fragment removal in in vitro fertilization. Fertil Steril 86: 321-324. [Crossref]
- Ebner T, Yaman C, Moser M, Sommergruber M, Polz W, et al. (2001) Embryo fragmentation in vitro and its impact on treatment and pregnancy outcome. Fertil Steril 76: 281-285.
- Halvaei I, Khalili MA, Esfandiari N, Safari S, Talebi AR, et al. (2016) Ultrastructure of cytoplasmic fragments in human cleavage stage embryos. J Assist Reprod Genet 33: 1677-1684. [Crossref]
- Chi HJ, Koo JJ, Choi SY, Jeong HJ, Roh SI (2011) Fragmentation of embryos is associated with both necrosis and apoptosis. Fertil Steril 96: 187-192. [Crossref]
- Alikani M (2007) The origins and consequences of fragmentation in mammalian eggs and embryos. Human Preimplantat Embryo Selection 51-78.
Editorial Information
Editor-in-Chief
John Livingston Powell
University of North Carolina School of Medicine
USA
Article Type
Case Series
Publication history
Received date: August 11, 2020
Accepted date: October 13, 2020
Published date: October 19, 2020
Copyright
©2020 Chae-Kim JJ. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Chae-Kim JJ, Waggener K, Gavrilova-Jordan L (2020) Defragmentation of in-vitro fertilization blastocyst stage embryos leading to rescued blastocyst expansion and clinical pregnancy. Clin Obstet Gynecol Reprod Med 6: DOI: 10.15761/COGRM.1000317
Corresponding author
Jennifer J Chae-Kim
Department of Obstetrics and Gynecology, Brody School of Medicine, East Carolina University, Medical Annex 165 – Vidant Medical Center, Greenville, NC, 27834, USA
E-mail : bhuvaneswari.bibleraaj@uhsm.nhs.uk

