Dendrobium, which is one of the frequently used medicinal herbs in Traditional Chinese Medicine (TCM) for treating diabetes, has aroused lots of attention on its potential alternative in the treatment of diabetes. This review aims to provide a comprehensive study on its phytochemical constituents, safety and pharmacological effects on treating diabetes and its complications with the underlying mechanisms uncovered. A comprehensive search of published medical literature from 2000 to 2020 was conducted by searching PubMed, Science Direct, Scopus, Web of Science and Google Scholar databases with keywords dendrobium, diabetes, phytochemistry, pharmacology and safety were used. Results showed that dendrobium exhibited anti-diabetic effects such as reducing gluconeogenesis, regulating lipid, protecting islet cells, anti-obesity, antioxidant and anti-inflammation on treating diabetes and its complications through the regulation of AMPK-GLUT4-PPARα; cAMP-PKA and Akt/Fox01; cRaf-MEK1/2-ERK1/2; IRS1-PI3K-Akt-Fox01/GSK 3β; MAPK; NF-κB; PI3k/Akt signaling pathway. The main chemical constituents of dendrobium species, which exert anti-diabetic, were polysaccharides. Most of the compounds of dendrobium species improved diabetes by antioxidant activity. No side effect of dendrobium species was reported in experimental studies. Therefore, our study suggested that dendrobium may offer a new potential alterative for prevention and treatment of diabetes and its complication. Well-designed clinical trials are needed for future studies.
dendrobium, diabetes, phytochemistry, pharmacology, safety
AC: Adenylate Cyclase; Akt: Protein Kinase B; Ala: Alanine; ALT: Alanine Transaminase; AR: Aldose Reductase; AST: Aspartate Aminotransferase; AUC: The Area Under the Curve; bFGF: Basic Fibroblast Growth Factor; BG: Blood Glucose; BUN: Blood Urea Nitrogen; BW: Body Weight; CA/CDCA: Cholic Acid/Chenodeoxycholic Acid; CAT: Catalase; CK: Creatine Kinase; Citr: Citrate; Create: Creatine; CREA: Creatinine; CTGF: Connective Tissue Growth Factor; DPPH, 2,2-Diphenyl-1-Picrylhydrazyl; ERG: Electroretinogram; FBG: Fasting Blood Sugar; FFAs: Free Fatty Acids; FN: Fibronectin; FINS: Fasting Insulins; Fox01: Forkhead Box Protein 01; GDH: Glucose Dehydrogenase; Gln: Glutamine; GLU: Glucagon; GLUT1: Glucose Transporter 1; GLUT2: Glucose Transporter 2; GLUT4: Glucose Transporter 4; G6Pase: Glucose-6-Phosphatase; GSK 3β: Glycogen Synthase Kinase 3 Beta; GSH: Glutathione; GSH-PX: Glutathione Peroxidase; GSP: Glucose Regulated Protein; HG: High Glucose; HIF-1α: Hypoxia-Inducible Factor 1-Alpha; HOMA-IR: Homeostasis Model of Assessment Insulin Resistance; Hs-CRP: High-sensitivity CRP; HW/BW: Heart to Body Weight Ratio; ICAM-1: Intercellular Adhesion Molecule 1; IFN-γ: Interferon-γ; IGF-1: Insulin-like Growth Factor 1; IL-1β: Interleukin-1β; IL-6: Interleukin-6; Ile: Isoleucine; INS: Insulin InsR: Insulin Receptor; iNOS: Inducible Nitric Oxide Synthase; KB: Ketone Body; LDL: Low-Density Lipoprotein; LDL-C: Low Density Lipoprotein Cholesterol; LDH: Lactate Dehydrogenase; Leu: Leucine; MDA: Malondialdehyde; MMP 2/9: Matrix Metalloproteinase-9; MT-1: Metallothionein-1; MyD88, Myeloid Differentiation Primary Response 88; 2-NBDG: 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2deoxyglucose; NF-κB: Nuclear-Factor Kappa β; Nqo1: NADPH Quinore Oxidoreductase-1; OGTT: Oral Glucose Tolerance Test; P-AMPK: Adenosine Monophosphate (AMP)-Activated Protein Kinase Phosphorylation; PEPCK: Phosphoenolpyruvate Carboxykinase; PDGF A/B: Platelet-Derived Growth Factor A/B; PGC1α: Alpha Susbunit of Peroxisome Proliferators-Activated Receptor-Gamma Coactivator-1; PKA: protein Kinase A; PI3K: Phosphoinositide-3-Kinase; PPARα: Peroxisome Proliferator-Activated Receptor α; ROS: Reactive Oxygen Species; SCr: Serum Creatine; SOD: Superoxide Dismutase; Tau: Taurine; TC: Total Cholesterol; Tch: Blood Lipids; TG: Triglycerides; TLR-4: Toll-Like Receptors; TNF-α: Tumor Necrosis Factor-α; T-SOD: Total Superoxide Dismutase; UA: Uric Acid; Val: Valine; VEGF: Vascular Endothelial Growth Factor.
Diabetes mellitus is characterized by high blood glucose level as a result of insufficient insulin for the body’s needs [1]. It is generally accepted that type 1 diabetes is due to the pancreas not producing enough insulin while type 2 diabetes is due to the cells of body not responding properly to the insulin produced [2]. Diabetes has become a worldwide major health-care problem as hyperglycemia increase the risk of complications such as diabetic retinopathy, diabetic cataract, diabetic nephropathy, diabetic cardiomyopathy etc [3].
However, diabetes is a chronic disease which cannot be cured and repaired because if the progressive reduction in beta-cell mass and inversible beta cell failure [4]. Due to the advance effect of drugs, current treatment for diabetes is not satisfactory [5]. As traditional Chinese medicinal herbs are relative cost-effective, multi-target and has low risk of advance effect, they become a potential candidate for diabetic drug development [6].
Dendrobium, which is one of the major genera of Orchidaceae, has both ornamental and medicinal value. There are more than 1400 species worldwide and are widely distributed in tropical and subtropical regions such as Asia, Europe and Oceania [7]. In China, there are about 80 kinds of dendrobium which are mainly distributed in the southwest, east and south of China [8].
Dendrobium is an important flower plants with high economic value. Its stems and flowers have been valued as precious food and herb medicine with healthy benefit and therapeutic effects. In traditional Chinese medicine, fresh of dry stems of some species of Dendrobium’s plants are harvested for medicinal purposes, collectively known as SHIHU. Its medicinal value has been first recorded in ancient Chinese medical book “Shen Nong’s Materia Medica” thousands of years ago [9].
Dendrobium is a valuable medicinal herb commonly used for nourishing yin and clearing heat in traditional Chinese medicine theory. It has the functions of moistening lung and benefiting stomach, clearing heat and brightening eyes, tonifying deficiency and strengthening body [10]. Thus, it can be used to benefit stomach, nourish body fluid, moisten lung and relieve cough. Recently, many researchers studied the chemical constituents and pharmacological effects of Dendrobium plants. It was found that the chemical constituents of Dendrobium plants include polysaccharides, alkaloids, bibenzyls, phenols, Phenanthrenes etc [11].
Several studies showed that Dendrobium has anti-aging, anti-cancer, digestion promotion, blood pressure reduction, cataract treatment and vasodilation effect [12]. At present, there are more than 50 kinds of medicinal Dendrobium such as dendrobium officinale, dendrobium huoshenense, dendrobium loddigesii, dendrobium aphyllum, dendrobium candidum, dendrobium crepidatum, dendrobium draconis etc [13].
In this review paper, the search was done in PubMed, Science Direct, Scopus, Web of Science and Google Scholar databases a 20-year period between 2000 to 2020 with keywords search of dendrobium, diabetes, phytochemistry, pharmacology and safety.
Recently, many researchers are interested in studying dendrobium species. However, there is no review paper focusing on the mechanism of therapeutic effects of dendrobium species on diabetes and its complications. Although some studies suggested that dendrobium can treat diabetes, there is lack of studies about the comprehensive mechanism of dendrobium’s anti-diabetic effects. Therefore, our review is the first study to fully understand the role of dendrobium in diabetes by studying its phytochemistry and pharmacological mechanisms on treating diabetes and its various complications. It also provides an interesting and illuminating insights to the readers who intend to perform clinical trials on dendrobium species in the future.
Phytochemistry of dendrobium: Nowadays, more than 50 compounds had been identified and isolated from dendrobium [14]. It was found that the chemical constituents of dendrobium are mainly polysaccharides, alkaloids, phenols, phenanthrenes and alkaloids [15]. The compounds which show anti-diabetic effects are listed in Table 1.
Table 1. The contents of compounds in different species of Dendrobium that exhibited anti-diabetic activities
Table 1 summarizes compounds which exert anti-diabetic effect in different species of dendrobium. Several chemical structures of these compounds involved in anti-diabetic activity are illustrated in Figure 1-4. These compounds include polysaccharide, phenanthenes, stilbenes, bibenzyl, polyphenol, indolizidine alkaloids. Most of these compounds showing anti-diabetic effects are polysaccharides. Besides, most of these compounds alleviate diabetes by antioxidant activity, implying a potential candidate for studying diabetes in the future.
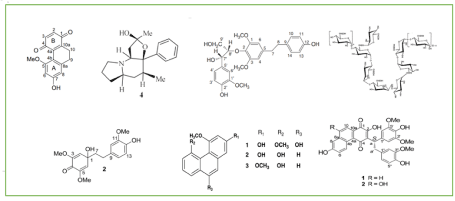
Figure 1. The contents of compounds in different species of Dendrobium that exhibited anti-diabetic activities
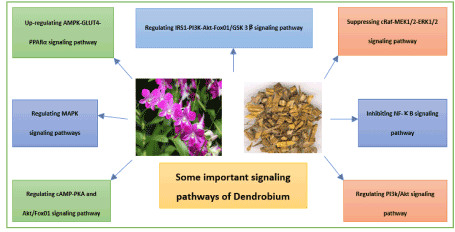
Figure 2. Animal studies about the antidiabetic effects of Dendrobium and its ingredients
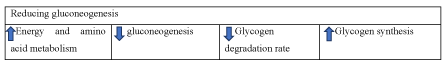
Figure 3. The chemical structures and names of compounds isolated from different species of Dendrobium that exhibited anti-diabetic activities

Figure 4. Medicinal plants Dendrobium used in treatment of diabetes and its complications with their mechanism of actions
Pharmacological activities of dendrobium in the management of diabetes
Reducing gluconeogenesis: In normal physiological conditions, liver glycogen synthesis and gluconeogenesis maintain a dynamic equilibrium [16]. However, when liver appears insulin resistance, which is defined as a pathological state that human body cannot respond to insulin normally, liver gluconeogenesis increases and hepatic glycogen synthesis decreases. Then, the balance between gluconeogenesis and glycogen synthesis is disrupted. After that, liver glycogen output increases and high blood glucose levels id resulted eventually. Thus, reducing gluconeogenesis is one of the targets to control blood glucose [17].
Dendrobium mixture, which includes 15 g dendrobium, 20 g astragalus, 8 g schisandra, 15 g pueraria, 15 g salvia, 15 g rehmannia and 8 g earthworms, improved insulin resistance and liver functions via regulating the PI3K/Akt signaling pathways. It is evidenced by Fox01, PEPCK, G6Pase decreased expression and InsR, PI3K, Akt increased expression. Thus, Dendrobium may improve liver glycogen and decrease blood glucose [18].
A water extract of dendrobium officinale was showed to up-regulate energy and amino acid metabolism as well as increase liver glycogen. It can reduce gluconeogenesis [19]. A study conducted by Hong-Yan Wang et al. showed that a polysaccharide from dendrobium huoshanense (GXG) could enhance glycogen synthesis and reduce gluconeogenesis via insulin-mediated IRS1-PI3K-Akt-Fox01/GSK 3βsignaling pathway. This study suggested that Dendrobium may reduce glycogen degradation rate by improving stability of liver glycogen structure [20]. In another study, a polysaccharide of dendrobium officinale (DOP) (100,200,400 mg/kg for 4 weeks) strengthened the fragile diabetic liver glycogen by inhibiting cAMP-PKA signaling pathway. Besides, it inhibited hepatic glycogen degradation and hepatic gluconeogenesis [21].
In brief, dendrobium has been evidenced to alleviate diabetes through reducing gluconeogenesis. The underlying mechanisms may be attributed to the regulation of PI3K/Akt, PI3K-Akt-Fox01/GSK 3β, cAMP-PKA signaling pathway (Figure 5).
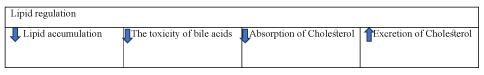
Figure 5. The diagram illustrates reducing gluconeogenesis of Dendrobium
Dendrobium alleviates diabetes through reducing gluconeogenesis, glycogen degradation rate and increasing energy and amino acid metabolism as well as glycogen synthesis.
Lipid regulation: Obesity is one of the causes of type 2 diabetes as lipid deposition in liver may lead to insulin resistance [22]. Besides, lipotoxicity may increase islet cell apoptosis and restrict the muscle’s usage of glucose capacity. Thus, it is important for diabetic patient to prevent dyslipidemia [23].
An extract of dendrobium nobile lindl. (DNLA) (15mg/kg for 18 weeks) was demonstrated to reduce the absorption of cholesterol by decreasing CA/CDCA ratio and increase the excretion of cholesterol by enhancing the taurine-conjugated bile acids [24]. A study conducted by Qiong Zhang et al. suggested that the extract of dendrobium finbriatium (100mg/kg,200 mg/kg for 4 weeks) could reduce lipid accumulation and lipotoxicity-induced hepatocyte apoptosis in rat. It also prevented islet cell apoptosis [25].
In a cell study of Xue-Wen Li et al., a shihurine-rich extract of D. loddigesii decreased the intracellular accumulation of oil droplets and triglycerides. It also increased 2-NBDG uptake of 3Ts-L1 cells [26]. Another study about dendrobium nobile lindl. revealed that an alkaloid of DNLA could increase lipid metabolism gene expression and decrease lipid synthesis regulator Srebp 1 [27].
Collectively, dendrobium alleviates diabetes by regulating the absorption and excretion of cholesterol. It can also reduce the toxicity of bile acids and lipid accumulation (Figure 6).
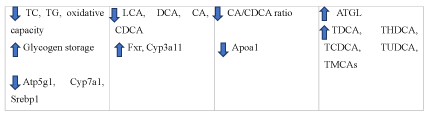
Figure 6. The diagram illustrates lipid regulation of Dendrobium
Dendrobium alleviate diabetes by reducing the lipid accumulation, the toxicity of bile acids, absorption of cholesterol as well as increasing excretion of cholesterol.
Protecting islet cells: The pancreas, which is a mixed gland formed by exocrine tissue, can synthetize and secrete inactive digestive enzymes. The endocrine tissue of pancreas is represented by the islets of Langerhaous consisted of alpha, gama, epsilon and beta cells [28]. Apoptosis is a form of beta cells death that happen in diabetes. A vitro study of a shihumine-rich extract of D. loddigesii showed that after 9 weeks of administration, the quantity of islet cells and the adipose cell size increased by up-regulating AMPK-GLUT4-PPARα signaling pathway. The expression of cleaved caspase-3 was also inhibited. Thus, it could prevent islet cell apoptosis [29].
In summary, dendrobium alleviates diabetes by protecting islet cells through the regulation of AMPK-GLUT4-PPARα signaling pathway. Dendrobium can protect islet cells and prevent islet cell apoptosis (Figure 7).
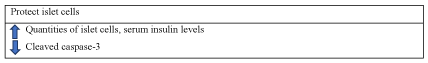
Figure 7. The diagram illustrates lipid regulation of Dendrobium.
Anti-oxidant: Oxidative stress is defined as an imbalance between oxidants and antioxidants in favor of the oxidants, leading to a disruption of redox signaling and control and/or molecular damage [30]. If excess reactive oxygen species is produced,β-cell maturation and apoptosis increases. Then insulin synthesis and secretion will be decreased. Both diabetes and obesity can increase the production of reactive oxygen species, resulting in oxidative stress [31].
A rich polyphenol extract of D. loggigesii (DJP) (25 mg/kg,50 mg/kg,100mg/kg for 8 weeks) was demonstrated to reduce the oxidative stress in db/db mice [32]. Another study using 1g/kg of the extract from dendrobium officinale to fed STZ rat for 5 weeks. Although there was no effect on blood glucose level and bodyweight, the glutathione peroxidase (GSH-PX) increased, implying the protective effects of this extract of dendrobium was related to antioxidant activity [33].
A cell study separated two polysaccharide fractions (DOPA-1 and DOPA-2) from stems of dendrobium officinale and tested the ability against H2O2-induced oxidative injury, DOPA-1 and DOPA-2 were found to suppress apoptosis and ameliorate oxidative lesions [34]. Another study about Alkaloids of dendrobium nobile lindl. (DNLA) showed that it could increase the expression of antioxidant gene MT-1 and Nqo1 in livers of mice through Nrf2-antioxidant pathway [27].
In short, dendrobium exerts anti-oxidant effects through Nrf2-antioxidant pathway. It also increases glucose metabolism genes and anti-oxidant genes. Dendrobium exerts anti-oxidant effects through increasing glucose metabolism genes and anti-oxidant genes (Figure 8).

Figure 8. The diagram illustrates anti-oxidant of Dendrobium
Anti-inflammation: Low-grade inflammation can lead to insulin resistance and is a main cause of Type 2 diabetes as pro-inflammatory macrophages may reduce the insulin sensitivity of liver, skeletal muscle and pancreatic β cells [35].
A rich polyphenol extract of D. loddigesii (DJP)was demonstrated to have anti-inflammation effect by reducing IL-6 and TNF-α [32]. Another study suggested that the extract of dendrobium fimbriation (DFE) could downregulate 588 differentially expressed genes (DEGs) and 74% of them were related to inflammatory, implying it may have anti-inflammation effect [25].
In brief, Dendrobium showed anti-inflammation effect by regulating NF-κβ signaling pathway. Dendrobium showed anti-inflammation effect by regulating NF-κβ signaling pathway (Figure 9).

Figure 9. The diagram illustrates anti-inflammation of Dendrobium.
Diabetic cardiovascular complications: Cardiovascular complications are notable causes of death in diabetic patients [26]. Diabetic cardiomyopathy is a serious diabetic complication as it is notable causes of death in diabetic patients. It is mainly manifested as myocardial dysfunction without other heart disease and may eventually lead to heart failure [36]. Chronic sustained hyperglycemia and insulin resistance may induce myocardial infarction and chronic pressure overload in diabetic patients with diabetic cardiomyopathy [37]. A dendrobium officinale extract (DOE), after 8 weeks of (75mg/kg, 150mg/kg, 300mg/kg) administration, was demonstrated that it could inhibit oxidative stress, cardiac lipid accumulation and pro-inflammatory cytokines in order to reduce cardiac fibrosis [38]. In a cell study conducted by Jing-yi Zhang et al., dendrobium officinale polysaccharides (DOY-GY) could exert cardioprotective effects on H2O2 induction-H9C2 cardiomyocytes through PI3K/Akt and MAPK pathways [39]. Collectively, dendrobium alleviates diabetic cardiovascular complications by reducing inflammation, cardiac fibrosis, cardiac oxidative stress, myocardial injury and apoptosis. The underlying mechanism may be associated with PI3K/Akt and MAPK pathways. Dendrobium alleviates diabetic cardiovascular complications by reducing inflammation, cardiac fibrosis, cardiac oxidative stress, myocardial injury and apoptosis (Figure 10).
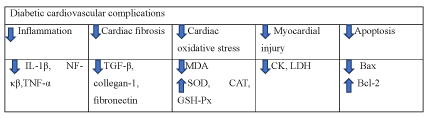
Figure 10. The diagram illustrates diabetic cardiovascular complications of Dendrobium
Diabetic nephropathy: Diabetic nephropathy is an important chronic micro-vascular complication of diabetes and may lead to end-stage renal disease [40]. Diabetic nephropathy is induced by diabetes and kidney dysfunction will be developed by disturbing renal tubular, glomeruli and its filtration barrier. Kidney functions continues to decline until end-stage renal failure was resulted [41]. Diabetes influences body’s metabolism and blood circulation, generating excess reactive oxygen species. which injure glomeruli and cause albuminuria [42]. The glomerular filtration barrier, which is composed of the fenestrated endothelium, the glomerular basement membrane and the epithelial podocytes, becomes more damaged in the progression of diabetic nephropathy. After administrating dendrobium candidum (0.2,0.4,0.8g/kg) for 8 weeks, it was found that it could improve pathological change in kidney and alleviate diabetic nephropathy by regulating VEGF, GLUT-1 and CTGF expression [43].
In vitro and vivo study of diabetic nephropathy, a methanolic extract of dendrobium monilfone (DM) was demonstrated to exert lipid lowering effect in HFD-induced obesity in mice as well as to inhibit the kidney cell damage induced by oxidative stress [44].
Another study using the extract of dendrobium officinale (5 ml/kg,10 ml/kg) for 4 weeks, result showed that it could alleviate diabetic nephropathy by preventing insulin resistance and reducing TLRs and inflammatory response [45]. Glucomannans, which is an extract of dendrobium officinale stem, could balance the disturbed glucose, lipid, amino acid metabolism and normalize the architecture of kidney corpuscle and tubular system after 4 weeks of drug (160 mg/kg) administration [46].
In summary, dendrobium alleviates diabetic nephropathy by reducing inflammation, oxidative stress, insulin resistance, renal dysfunction and diabetic kidney lesion. Dendrobium improves diabetic nephropathy by reducing oxidative stress, renal dysfunction, insulin resistance, inflammatory response and diabetic kidney lesions (Figure 11).
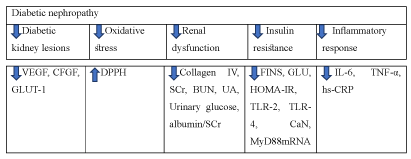
Figure 11. The diagram illustrates diabetic nephropathy of Dendrobium
Diabetic retinopathy: Diabetic retinopathy is a common diabetic complication. It is characterized by hard exudates, microaneurysms, macular edema and retinal hemorrhage [47]. The ethanol extract of D. Chrysotoxum (30 mg/kg,300 mg/kg for 1 month) was demonstrated to breakdown the blood retinal barrier, inhibit retinal inflammation and prevent the decrease of tight junction protein such as occludin and claudin-1 [48]. In vivo and vitro study by Zengyang Yu et al., erianin, which was extracted from dendrobium chrysotoxum Lindl., could inhibit retinal neoangiogenesis by abrogating HG-induced VEGF expression. It could also block ERT1/2-mediated HIF-α activation in retinal endothelial and microglial cells [49]. Another study also found that the ethanol extract of dendrobium chrysotoxum Lindl could alleviate retinal angiogenesis and ameliorate retinal inflammation by inhibiting NFκβ signaling pathway [50]. To conclude, dendrobium alleviates diabetic retinopathy by reducing retinal neoangiogenesis and tight junction protein. It can also reduce pro-angiogenic factor and inflammation. Dendrobium alleviates diabetic retinopathy by reducing inflammation, retinal neoangiogenesis, tight junction proteins and pro-angiogenic factor (Figure 12).
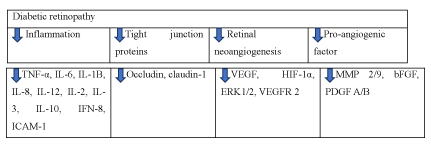
Figure 12. The diagram illustrates diabetic retinopathy of Dendrobium
Diabetic Cataract: Cataract is the leading cause for impaired vision and blindness in patients with diabetes [51]. Hyperglycemia-associated increase in osmotic pressure and oxidative damage are the main causes for the development and progression of diabetic cataract [52].
In a cell study conducted by Jie Wu et al., a gigantol from dendrobium chrysotoxum Lindl. was showed to inhibit AR gene expression and aldose reductase in Human lens epithelial cells (HLECs) [53]. Another study showed that gigantol from dendrobium aurantiacum var dennam could prevent galactose-induced damage to the rat lens by repressing the gene expression and activity of AR & iNOS. It could also delay lens turbidity and keep lens transparent [54].
To sum up, dendrobium alleviates diabetic cataract by reducing damage of osmotic pressure stress and oxidative damage. Dendrobium improves diabetic cataract by reducing oxidative damage and damage caused by osmotic pressure stress (Figure 13).
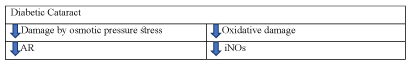
Figure 13. The diagram illustrates diabetic cataract of Dendrobium
Safety of Dendrobium: In the “Shen Nong’s Materia Medica”, dendrobium is classified as “Top-tier” medicinal herb which is regarded as an effective medicinal herb without observable toxicity. The safety of medicinal herb is important because the intake of heavy metal elements into human body is harmful. One study conducted by Yingdan Yuan et al. showed that the dosage of 12g d-1 dendrobium, which is prescribed in the Chinese Pharmacopoeia 2010 edition, is in accordance with the recommended daily intake of trace elements recommend by the Food and Drug Administration of the United States [55]. Another study by Li-Chan Yang et al. assessed the 90 days oral toxicity and genetic safety of the aqueous extract of Dendrobium Taissed Tosnobile in 90 sprague-dawley (SD) rats, no abnormal changes were observed in clinical signs and body weight. Also, no significant difference between treatment and control group was found in biochemistry parameter, urinalysis and hematology throughout the study period [56-65]. Therefore, when taken according to the dosage prescribed by the pharmacopoeia does not cause any adverse effects and trace elements poisoning.
Dendrobium is one of the most frequent used medicinal herbs for treating diabetes in TCM clinical practices. Table 2 and Table 3 summarizes its hypoglycemia effects in animal studies and cell-based studies. In recent studies, there are more than 50 compounds isolated and identified from dendrobium. The types of compounds, which show anti-diabetic activities, include polysaccharide, phenanthenes, stilbenes, bibenzyl, polyphenol, indolizidine alkaloids. Most of the compounds showing anti-diabetic effects are polysaccharides. The signaling mechanisms of dendrobium treating diabetes may be involved in the regulation of AMPK-GLUT4-PPARα; cAMP-PKA and Akt/Fox01; cRaf-MEK1/2-ERK1/2; IRS1-PI3K-Akt-Fox01/GSK 3β; MAPK; NF-κB; PI3k/Akt which are illustrated in Figure 2.
Table 2. Animal studies about the antidiabetic effects of Dendrobium and its ingredients













