Sodium dichloroisocyanurate (NaDCC) is a broad-spectrum disinfectant agent often used in the health system of the province of Quebec in Canada, particularly in the cases of Clostridium difficile. To be effective against bacteria and the spores, an adequate concentration of HOCl- molecules is required. In order to find out the efficiency of the disinfection, NaDCC was applied to four types of surfaces commonly found in the hospital environment such as mattress cover, melamine, glass and Formica®. Three application modes of the product including one step, two steps and three steps were used. Using a Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS), semi-quantitative measurements of the concentration of the main molecules of interest (HOCl-, OCl-, NClH, NClH2) were conducted. The results revealed that the nature of the surfaces and the use of a quaternary ammonium mixture for cleaning can participate in the chemical reactions involved and thereby tend modulate the concentration of disinfectant molecules from NaDCC. This modulation, in general, was related to the presence of nitrogen compounds from quaternary ammoniums, the surface or both, example on the mattress cover with a film of quaternary ammoniums. In this work, we found that the melamine would be the hard surface that would be potentially the most difficult to disinfect.
Clostridium difficile, spores, disinfection, sodium dichloroisocyanurate, TOF-SIMS
In healthcare facilities, surface disinfectants play a major role in controlling environmental transmission and spread of nosocomial infections by contact with hitherto “noncritical” inanimate/environmental surfaces [1]. Mafu [2] showed that attached cells which are intimately associated with the inanimate material may be less susceptible to cleaning processes due to the boundary layer and the secretion of extracellular coatings. The ability of microorganisms to become more resistant to sanitizers and other antimicrobial agents once attached to the surfaces were also documented [3]. Therefore, control of this nosocomial infection requires an appropriate application of a strategy that optimize reducing the environmental burden [4]. This strategy should include a product with sporicidal activity against Clostridium difficile spores. The resistance of the spores to the majority of disinfectants results from its dehydration state and its wall, which is composed of several layers of different proteins [5].
The sodium dichloroisocyanurate (NaDCC) is employed against bacteria and spore of Clostridium difficile in hospitals in the province of Quebec. The concentration of the sporicidal molecules to be optimal in order to significantly reduce the infectious risk. In the case of sodium dichloroisocyanurate (NaDCC), the sporicidal molecules produced during solutioning are mainly the HOCl- molecules. According to Perez [6], an oxidizing agent solution such as a chlorine solution is effective against C. difficile spores at the concentration greater than 3000 ppm. Other authors reported a concentration of 5000 ppm chloride molecules in solution was required to be effective [7,8].
Thus, the effectiveness of many disinfecting agents depends on a number of factors that may have a significant effect on the end result. They include length of exposure, temperatures, nature of the organism, product concentration, amount of organic matters [2]. However, we tend often to neglect another parameter in this equation, particularly the chemical composition of the surfaces and cleaning product film. The surfaces can react chemically with the latter [9-11]. It is in this context that we studied the impact of the chemical interaction between different surfaces and the application of sodium dichloroisocyanurate (NaDCC) in the production or loss of HOCl- molecules.
The aim of this study was to evaluate trending the chemical disinfecting products composition between the different surfaces and the application of sodium dichloroisocyanurate (NaDCC) during surface sanitizing. In this work, four common surface types found in hospital environments such as melamine, glass, bed liners made of 2-methylprop-2-enoate from methyl (PMAM) and arborite were used.
Test surfaces
Four common surface types found in hospital environments, namely glass (borosilicate cover slips for optical microscopy, VWR International Inc., West Chester, PA), melamine (Bel-Trim, Canada) and Arborite, also known as Formica (Compagnie Bélanger, Quebec, Canada and a piece of mattress covers in acrilyc (polymetacrylate methyl (PMAM)) were used (Table 1).
Table 1. Test Surfaces
Surface |
Composition |
Melamine |
1,3,5-triazine-2,4,6-triamine combined with formaldehyde urea |
Polymethyl methacrylate (PMMA) |
Methyl 2-methylprop-2-enoate |
Glass |
SiO2 (the most important molecule) |
Arborite |
The laminated kraft paper is impregnated with phenolic resin and melamine formaldehyde [12] |
For each surface and for each treatment, a square was cut resulted to a total of 24 squares which corresponds to 24 samples. Each sample was an area of 40 × 40 μm2.
Preparation of chemicals
The commercial disinfection products used in this study was sodium dichloroisocyanurate (NaDCC). This product is used in Quebec’s health system. To represent the actual situation in hospitals environment, the NaDCC solution was prepared with water from the municipal water supply system of Montreal. For all the trials, water was collected in the same sampling operation and was used quickly to limit differences in the concentration of any molecules that may have been presented. In all the trials, the products were applied to the surfaces with a cotton cloth (Compagnie de produits Sany, Joliette, QC, Canada).
Test procedures
Control
Using surfaces to which products were not applied (control) made it possible to determine the main chemical components of the surfaces and to make distinctions between control and treated surfaces.
Ammonium quaternary
Before disinfection it is important to cleaning the surface like in the hospital. Therefore a quaternary ammonium (Quat) solution (standard 1/62 dilution as recommended by the manufacturer) was applied to each tested surface (glass, melamine, mattress cover or Arborite) using a cotton cloth soaked in the product. To ensure that the test surfaces were properly wetted, the cotton cloths were wiped across the surfaces two times. The surfaces were air-dried for at least 10 min prior to any examination. The contact time of 10 minutes was the maximum time required due to make availability of the hospital room following the patient’s a departure. The surfaces were then tested using time-of-flight secondary ion mass spectrometry (TOF-SIMS) on an ION-TOF SIMS IV instrument (ION-TOF GmbH, Münster, Germany).
Sodium dichloroisocyanurate (NaDCC)
For the disinfection, to each surface NaDCC solution was applied at the concentration of 5000 ppm (identical to the concentration used in Quebec) using a cotton cloth soaked in the product solution. The surfaces were air-dried for at least 10 min prior to any examination.
The tests of surface cleaning-disinfections were conducted according to one, the two-step and three-step methods.
One step
The product was applied to all surfaces using cotton cloths and air-drying for 10 minutes prior to the analysis.
Two-step cleaning-disinfection
In this part, a Quat solution (1/62) was applied to the surfaces using cotton cloths. To ensure better wetting of the inert material, the cotton cloths soaked in the chemical solution and wiped across the surfaces two times before the air-drying for 10 min. Similar procedure was used to apply the NaDCC solution at concentration of 5000 ppm prior to analysing with TOF-SIMS.
Three-step cleaning-disinfection
The three stages cleaning-disinfection is used in some Quebec’s hospitals for the terminal disinfection of a case of C. difficile. The cleaning-disinfection comprises of application of quaternary ammonium (like two-step cleaning-disinfection), followed by rinsing with tap water and then using the solution of NaDCC.
For this step, a Quat solution was applied with cotton cloths to the surfaces of glass, melamine and arborite. The cloths were wiped across the surfaces two times and left to air-dry for 10 min. Then, the surfaces were cleaned with cotton cloths soaked in warm tap water. For NaDCC, the solution at 5000 ppm was applied with cotton cloths. In both cases, ToF-SIMS was used to examine the surfaces.
Equipment
The TOF-SIMS instrument available at the École Polytechnique de Montréal was used for the physicochemical characterization of the various surfaces [1,13], Biophy Research, Time-of-Flight, TOF-SIMS). TOF-SIMS data were acquired using a TOF-SIMS IV instrument (ION-TOF GmbH, Münster, Germany).
The measurements
The measurements were taken with an ION-TOF SIMS IV system (ION-TOF GmbH, Münster, Germany). The system pressure was 5 × 10-9 torr. The source was monatomic and consisted of a pulsed beam of gallium 69. The mass/charge ratio of the detected ions was measured. A voltage of 15 V and a current of 0.5 µA were used to remain in static mode. The surface was therefore not modified by the bombardment, and the lifetime of a monolayer is a few hours, so in dynamic mode the surface is broken in a few nanoseconds and the current is 1 A/cm2. A dose less than 1011 ions/cm2 had to be respected. The verified parameters were as follows: a current of 2.0 pA, a hydrogen resolution less than 0.8, and a silicon resolution greater than 8,000. The primary ions were pulsed at a width of 30 ns and a current of 2.0 pA with a primary ion dose less than 5 × 1011 ions/cm2, which was well below the threshold of 1 × 1013 ions/cm2 required to remain in static mode. Electrostatic charges were neutralized by a low-energy electron source. The secondary ion spectra were acquired on a surface area of 40 × 40 µm with 128 × 128 pixels (one pulse per pixel), on only one position per sample. The measurements were conducted, in first, at both polarities, i.e. positive (SIMS+) and negative (SIMS−).
The measurements were conducted in three places for each treated surface and the control. The spectres of three measures were compared to standard spectres. The closer spectre to the standard spectre was considered and used as spectre for the identification and quantification. It is about a semi-quantitative measure of the concentration present. The first step consisted of analysing the chemical composition of the three surface types under study. In the second step, the surfaces were characterized individually, first with NaDCC and second with Quat only. The elements that were present characterized the substance or its interaction with the surface. All the molecules of interest to this study were of negative polarity. For that reason, the results obtained by positive SIMS were not considered.
The retained molecules were hypochlorite molecules (ClO−) and hypochlorous acid (HOCl-) since they are characteristic of the oxidizing (ClO−) and bactericidal (HOCl-) power of NaDCC. It should be noted that the method that was used did not allow us to evaluate the hypochlorous acid molecule evaporation rate. The results of the semi-quantification of the number of molecules were normalized using the number of detected Cl2 molecule as a reference. This is justified because this molecule is shared by the prepared NaDCC and QUAT solutions. The preparation of these products involves, usually, a dilution with water from the municipal water that contains molecules Cl2. The presence of these molecules can then be added to those present in the process. After calibration, the molecules of interest were identified from their molecular masses. For the control substrates, the same molecular masses were explained by other combinations of periodic elements table, selected based on prior knowledge of the substrates chemical composition.Using the available software provided from the manufacturing company, the areas under the peaks were calculated by integration the region delimited by the curve. Therefore, the number of molecules at the spot where obtained. The molecules of interest including Cl2, ClO−, HOCl-, NClH and NClH2 were identified. Both NClH and NClH2 are the result of the reaction between Quat residu and chloride molecules, moreover, the formation of amines reduces the potential disinfectant.
Because of their respective chemical compositions, the surfaces react differently to the applied treatments. Therefore, the quantities of molecules of germicidal groups that are received by TOF-SIMS detector do not allow a comparison between different surfaces [11]. It is therefore necessary to compare the quantities of different germicidal molecules generated for the same surface.
Similarly, this study relies only on qualitative and non-quantitative analyses between treatments. The number of molecules received by the detector is a variable that cannot be quantified due to certain physical phenomena, it will be necessary to stop the reports orders of magnitude that will be sufficient to determine if one molecule predominates over another [11].
The results of the semi-quantification for different molecules of interest are shown in Figures 1 at 4. The figures show major variations in the concentrations of the main components under study depends on the nature of surface and cleaning technique. They are intensities of spectrum peaks in relation to the approximate number of molecules present on surfaces according to groupings and treatments each time. That's why we cannot apply statistical processing to our data (Figure 1).
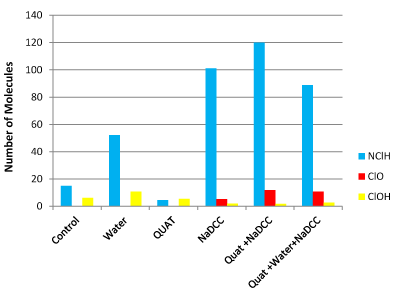
Figure 1. Arborite.
Arborite®
Comparing to the control, this surface reveals that there are few chloramines in the composition of the surface of Arborite®. The addition of tap water can be caused a possibly increase of peak intensity for NClH and HOCl-. It was also observed that the addition of NaDCC induced significant increase in the presence of chloramines and to a lesser extent the presence of ClO-. Uploading the ammonium quaternary product (QUAT) only has little effect on the reading of the peak intensity for the amines. The presence of chloramines when there is maximum combination with QUAT. The production of ClO− is slightly stimulated by the addition. However, we note that the peak intensity with the technique in three steps outside to reduce the presence of chloramines may seem to have an impact on the concentration of hypochlorous acid. The applications on this surface don’t show an important concentration of molecules HOCL- (Figure 2).
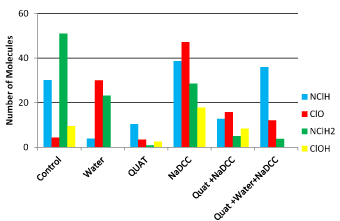
Figure 2. Melamine.
Melamine
It was found that the composition of melamine is composed of NClH and NClH2. We can also observe both ClO− and HOCL- molecules on the surface. The addition of tap water caused possibly an increase in ClO− molecules and the use of cleaner-disinfectant has little impact on reading. Against by the addition of NaDCC only has an effect that stands out in the presence of ClO− and HOCL- and chloramines while the combination QUAT- NaDCC and also has an effect but to the lesser extent. Rinse with tap water to the third step resulted in the disappearance of HOCL- molecules while NClH ions dominate the use of a three-step strategy on this surface (Figure 3).
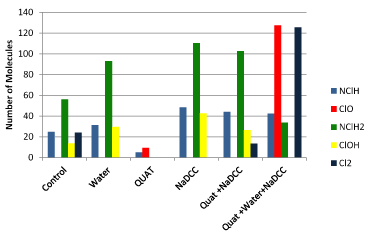
Figure 3. Mattress cover.
Mattress covers
The use of water also led to an increase in the presence of chloramine and the disappearance of HOCL- but chlorine and hypochlorous acid. The increase of chloramines is more than double the increase in the hypochlorous acid. Just as in the case of the other two surfaces QUAT only produces little amines and molecules disinfectants. For cons, the use of NaDCC provides a significant peak of NClH2 and HOCL-. The two-step technique differs with NaDCC mainly by the presence of Cl2. The three-step technique with the rinsing phase between the application and that of the QUAT and NaDCC has an intensity peak of significant hypochlorous molecules of ClO−-and chlorine Cl2 molecule. However, hypochlorous acid disappears and dichloramines (NClH2-) decrease in intensity (Figure 4).
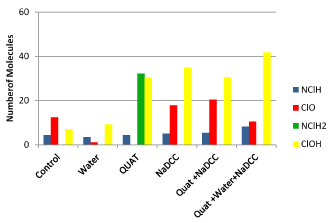
Figure 4. Glass.
Glass
Observed on this surface the presence of molecules and ClO-, NClH2, HOCL-. The same type of situation occurs with the tap water. Uploading quaternary ammonium on the glass causes a significant increase in the peak intensity molecules of NClH2 and HOCL-. It is a phenomenon that has not been observed on other surfaces. The treatments with single NaDCC and QUAT favor the presence of molecules HOCL- and that of ClO-and there are little chloramines. The three-step strategy clearly favors the production of molecules HOCL- and there are few molecules chloramines. This surface chemically is the easier to cleaning and disinfecting against spores of Clostridium difficile.
Some hospitals use as a source of hypochlorite molecules from a solution of sodium dichloroisocyanurate (NaDCC) to fight against Clostridium difficile. Indeed, it is the NaDCC when dissolved in water allows the production of hypochlorous acid and hypochlorite molecule as sodium hypochlorite.
C3Cl2N3O3Na + 2H2O  C3H3N3O3 + HOCL- + Na+ + ClO-
C3H3N3O3 + HOCL- + Na+ + ClO-
The effectiveness of hypochlorous acid is linked with the absence of an electrical charge as well the molecule’s chemical form, which resembles that of water. The cytoplasmic membrane allows this molecule to go through with water and hypochlorous acid oxidise the enzymatic activity inside the bacteria [14]. For the C. difficile spore, according to Fuzukaki, the concentration of OH− ions plays a role in destruction of protein membranes and induce lysis of the spore. Without that destruction of the membranes, the negatively charged hypochlorite ions would be repelled by the anions in the membranes [15].
In a disinfectant cleaning procedure, the presence of organic soiling should normally first make a cleaning before disinfection because the organic matter on the surfaces can reduce the efficacy of ions chloride [16]. In Quebec, quaternary ammoniums (Quat), a cationic surface-active agent with detergent action, is generally used in hospital facilities [17].
The Quat produces on the surface a dry film containing nitrogen molecules. When applied to this film dried a chlorine solution there can form chloramines (NHCl2, NH2Cl and NCl3). These are recognized as having a potential lower than the disinfectant chloride ions but not sporicide [18]. Hence the importance of studying the insertion of a rinsing steps between the application of cleaner and disinfectant. It should be noted that in addition to reacting with the dried film of Quat, the chlorine solution can also react with nitrogen molecules contained in some surface such as melamine and cover mattress (Figure 2,3) [11]. Hypothetically, it was found that the nitrogen product on the mattress cover for the control (Figure 3) because this is not a new one. Despite the synthetic composition of the mattress cover, the latter looks like a woven surface. It would therefore be more difficult to eliminate the Quat film that can be housed there.
The results shown in figures 1–4 reveal that the peak intensity of desired molecules are highly variable depending on the nature of the surface. As observed in these figures, the chloramines ions are generally present on any surface and the type of treatment, even with tap water. Treated tap water contains chloramines and chloride molecules [18]. The cleaning products, disinfectants, tap water and all surfaces interact chemically and thus modulate the generation of potential sporicides molecules. This chemical interaction allows an increased presence of chloramines and chlorinated lower intensity molecules on the surfaces of melamine and formica surfaces compared to the other two. A subsequent study using sodium hypochlorite also shows an increased production of chloramines on melamine compared to other surfaces [18].
As shown in figures 1 and 2, regardless of the technique used, the results show that NaDCC is not really creating a sporicidal chemical environment compared to using a three-step technique in figures 3 and 4. Despite this situation in figures 1 and 2, it still remains that rinsing with water in a three-step technique is a mechanical action that allows additional removal of bacteria and organic residue while preparing the surface for the disinfectant. The suspension of spores in water makes the products more effective compared to the application of the products directly on a dry surface. The presence of water rehydrates the spores which facilitates the penetration of chlorine ions within the spore and thus impair its ability to regenerate [19].
However, we observed that a relatively stable chemical surface such as glass does not have a molecule composition and amino and chlorine ions dominate in intensity regardless of the type of treatment 1, 2 or 3 steps. The presence and importance of the intensity peaks of chlorine molecule on the surface can theoretically achieve an environment that facilitates sporicidal activity. This observation is quite important especially for critical care areas. The presence of materials containing amino ions could be problematic in these critical areas where there is risk of environmental contamination by Clostridium difficile spores.
In conclusion, this work mainly permitted studying of the chemical nature influence of the surfaces and the cleaning product trend the production of HOCl-, microbiocide and sporicidal molecules when a solution of NaDCC was applied to inert the surface of materials. This work has revealed that the production of HOCl- from NaDCC tends to be modulated by the nature of the surfaces, such as the glass surface and the mattress pad. We have also found that the technique and cleaning-disinfection products can modulate the concentration of HOCl- molecules. A three-step technique on a surface chemically stable and without the presence of nitrogen molecule tend to demonstrate a greater sporicidal and microbiocide potential with the concentration of HOCl - compared to other types of surfaces. Currently in hospitals, chemical composition of surfaces is rarely considered in relation to the risk of infection. In this work, melamine would be the hard surface that would be potentially trend the most difficult to disinfect. This study shows that it may be important to take account of the nature of the surfaces and the cleaning product when we want to develop the good proceeding against bacteria and spore.
The authors would like to thank Dr. Edward Sacher and Ms. Suzie Poulin from the LIAB and the of Groupe Hygiène et salubrité of the Service des activités de soutien et du partenariat du Ministère de la Santé et des Services sociaux du Québec for their collaboration, the financial Support of the Ministère de la Santé et des Services sociaux du Québec for, which made this project possible. Finally, the authors would like to thank Drs Alberta Aryee and Reza Zareifard for editing this article.
RM conceived, designed the experiments and wrote the main manuscript text. AAM wrote the main manuscript text. DAB, MT, DM, performed the experiments and analysed the data. LY and GP supervised the experiments works and reviewed the manuscript.
There is no conflict of interest to declare.
- Mafu AA, Massicotte R, Pichette G, Ahmad D (2015) Importance of mechanical action in a terminal disinfection process for decontamination of Clostridium difficile spores on hospital inert contact surfaces. Int J Infection Control 11: 1-9.
- Mafu AA, Roy D (2011) Disinfecting and sterilizing agents used in food industry. In: R.G. Labbé and S. Garcia (Ed.), Guide to Foodborne pathogens. USA; Wiley-Interscience, pp. 315-332.
- Mafu, AA, Roy D, Goulet J, Savoie L, Roy R (1990) Efficiency of sanitizing agents for destroying Listeria monocytogenes on contaminated surfaces. J Dairy Science 73: 3428–3432. [Crossref]
- Worsley MA (1998) Infection control and prevention of Clostridium difficile infection. J Antimicrob Chemother 41: 59-66. [Crossref]
- Paredes-Sabja D, Shen A, Sorg JA (2014) Clostridium difficile spore biology: sporulation, germination, and spore structural proteins. Trends Microbiol 22: 406-416. [Crossref]
- Perez J, Springthorpe VS, Sattar SA (2005) Activity of selected oxidizing microbicides against the spores of Clostridium difficile: Relevance to environmental control. Am J Infect Control 33: 320-325. [Crossref]
- Massicotte R, Hygiene and Sanitation Group for the Control of Nosocomial Infections Department of Health and Social Services (2008) Disinfectants and Disinfection in Hygiene and Sanitation: Fundamental Principles, Communications Branch of the Department of Health and Safety Quebec Social Services. pp: 77.
- Wilcox MH (2003) Comparison of the effect of detergent versus hypochlorite cleaning on environmental contamination and incidence of Clostridium difficile infection. J Hosp Infect 54: 109-114. [Crossref]
- Mireles LK, Dayan J, Massicotte R, Dagher F, Yahia LH (2016) Interactions of active compounds of disinfectants on metallic and polymeric hospital surfaces. Clinical Medical Investigations 1: 39-47.
- Dayan J, Mireles LK, Massicotte R, Dagher F, Yahia LH (2016) Effect of disinfectants on wettability and surface tension of metallic and polymeric surfaces found in hospitals. Clinical and Medical Investigations 1: 48-55.
- Massicotte R, Ginestet P, Yahia LH, Pichette G, Mafu AA (2011) Comparative study from a chemical perspective of two- and three-step disinfection techniques to control Clostridium difficile spores. Int J Infect Control 7: 1-8.
- Laminating Materials Association (2001) Glossary of terms. Laminating Materials Association. Hillsdale, New Jersey, USA pp: 20.
- Cizaire L, Martin JM, Le Mogne T, Gresser E (2004) Chemical analysis of overbased calcium sulfonate detergents by coupling XPS, ToF-SIMS, XANES, and EFTEM. Colloids Surfaces A: Physicochemical and Engineering Aspects 238: 151-158.
- Fuzukaki S (2006) Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol Sci 11: 147-157. [Crossref]
- Water Treatment Solutions Lenntech, Les biocides. Available from: http://www.lenntech.fr/biocide.htm#Quaternary%20ammonium%20salts
- Ungurs M, Wand M, Vassey M, O'Brien S, Dixon D, et al. (2011) The effectiveness of sodium dichloroisocyanurate treatments against Clostridium difficile spores contaminating stainless steel. Am J Infect Control 39: 199-205. [Crossref]
- Rizk-Ouaini R, Ferriol M, Gazet J, Saugier-Cohen Adad MT (1986) Oxidation reaction of ammonia with sodium hypochlorite. Production and degradation reactions of chloramines. Bull Soc Chim de France 4: 512-521.
- Lee W, Westerhoff P (2009) Formation of organic chloramines during water disinfection – chlorination versus chloramination. Water Res 43: 2233-2239. [Crossref]
- Maillard JY (2011) Innate resistance to sporicides and potential failure to decontaminate. J Hosp Infect 77: 204-209. [Crossref]




