Introduction: Modern techniques of immediate breast reconstruction after mastectomies for breast cancer gives excellent cosmetic results and improve quality of life. However, it is perceived that immediate breast reconstruction may prolong recovery and can result in complications delaying adjuvant therapy. We aim to determine if there is such delay in the United Kingdom beyond the 31 days recommended by the National Institute for Health and Care Excellence.
Materials and methods: All patients who underwent mastectomy for breast cancer from January 2009 to August 2014 and received adjuvant treatment were categorised into three groups – mastectomy, implant / expander and flap. The primary end point was the time interval from the definitive surgical procedure to the start of adjuvant therapy.
Results: Of the 192 patients (64 per group) analysed, mastectomy patients were significantly older, smokers and with higher nodal status (p < 0.05). The groups were comparable with respect to other clinicopathological factors (p > 0.05).
Six patients from implant group, one patient from flap group and none from mastectomy group started their adjuvant therapy within 31 days. The mean interval from surgery to start of adjuvant therapy was 63.2 days (33–202) in mastectomy group, 52.82 days (26–136) in implant group and 50.61 days (29–89 days) after flap procedures (p = 0.004).
Conclusion: Our study shows a delay in initiating adjuvant therapy in keeping with published literature. The reasons could be multifactorial including delay in service provision. This delay is statistically significant in the mastectomy-alone patients, perhaps because they were older, and smokers compared to the other groups. We believe treatment pathways and multidisciplinary clinics will circumvent these concerns.
breast cancer, mastectomy, reconstruction, adjuvant therapy, breast implant, free flaps
Breast cancer is one of the most common cancers in women worldwide, with 55,122 new cases of invasive breast cancer being diagnosed in the United Kingdom in 2015 [1]. The overall outcome in early stage breast cancer has improved considerably over the last two decades with a current overall 5-year survival of 87% in the United Kingdom. The reason for this improved outcome is multifactorial with advances in surgical techniques, adjuvant therapies, breast screening and public awareness as major contributing factors. With increase in life expectancy, the quality of life gains more importance. One vital aspect in improving the quality of life after mastectomy for breast cancer is reconstructing the breast.
Immediate breast reconstruction (IBR) after mastectomy for breast cancer has been shown to have a positive influence over the delayed reconstruction on body image and sexuality, improving psychosocial well-being, reducing anxiety levels, resulting in excellent patient satisfaction and improving self-esteem and quality of life [2-6]. It may also avoid further admissions for planned surgical procedures [7].
A number of studies have shown that reconstruction is oncologically safe after mastectomy even in advanced disease [8-10]. Current UK oncoplastic breast reconstruction guidelines [11] recommend that oncoplastic breast surgery is discussed in 100% of patients requiring a mastectomy. Skin sparing mastectomy (SSM) has been shown to be oncologically safe with low local recurrence rates [12] and combined with immediate breast reconstruction provides superior aesthetic outcome with less disruption to the patient’s lifestyle [13]. The United Kingdom national mastectomy and breast reconstruction audit 2011 [14] has shown that 3389 (21%) patients underwent immediate breast reconstruction out of the 16485 patients who had mastectomies during their study period of 15 months.
Modern techniques of IBR like Deep Inferior Epigastric Perforator (DIEP) flaps and Acellular Dermal Matrix (ADM) based implant reconstruction give excellent cosmetic results. There is a perception that these complex procedures may have prolonged recovery and can result in significant complications, which may unduly delay the initiation of adjuvant therapy or lead to its omission altogether. This is because adjuvant therapy after breast cancer surgery has shown to produce a significant survival advantage and reduction in local recurrence in selected patients [15,16].
In a meta-analysis, 6 months of anthracycline-based poly-chemotherapy reduced the annual breast cancer death rate by about 38% for women younger than 50 years of age and by about 20% for those of age 50–69 years irrespective of the use of tamoxifen and of oestrogen receptor (ER) status, nodal status, or other tumour characteristics [15]. In another large meta-analysis done by the Early Breast Cancer Trialists’ Collaborative Group [16], for 1314 women with axillary dissection and one to three positive nodes, radiotherapy reduced locoregional recurrence (p < 0.00001), overall recurrence (RR 0.68, 95% CI 0.57–0·82, p = 0.00006), and breast cancer mortality (RR 0.80, 95% CI 0.67–0.95, p = 0.01).
The optimum duration to start of adjuvant therapy after breast cancer surgery is not clearly defined. The National Institute for Health and Care Excellence, UK (NICE) has recommended that adjuvant therapy should be started by 31 days of completion of definitive surgery [17]. American Society of Clinical Oncology (ASCO)/National Comprehensive Cancer Network (NCCN) quality measures recommends adjuvant chemotherapy within 120 days of diagnosis for women aged less than 70 years with stage II or stage III hormone receptor-negative breast cancer [18]. The 120-day threshold was selected as a “reasonable estimate of the time required to deliver the preceding components of therapy that would not jeopardize outcome” [18].
Available evidence looking at the delay between IBR and adjuvant therapy has given mixed results and used data before the widespread use of the above modern methods of reconstruction. Most studies evaluated patients on an intention to treat basis (who received chemotherapy) rather than those for whom chemotherapy was indicated. This can miss patients who did not receive adjuvant therapy because of complications of breast reconstruction. Further, most studies only compared mastectomy with IBR without dividing the IBR group into implant based and free flap groups which are associated with different complications and recovery times.
Overall there is no clear consensus on the optimum time to give adjuvant chemotherapy or radiotherapy after surgery and mixed evidence on the effect of early initiation of adjuvant therapy after breast cancer surgery.
The aim of the study is to determine if modern methods of post-mastectomy immediate breast reconstruction delay the start of adjuvant chemotherapy or radiotherapy in the United Kingdom based on NICE recommendations and if there is a difference between mastectomy, implant procedures and flap-based procedures in the time to start adjuvant therapy.
A retrospective audit was conducted in four hospitals of the Essex cancer network for the period from January 2009 to August 2014. All patients who underwent mastectomy for breast cancer and received adjuvant chemotherapy or radiotherapy were grouped into mastectomy without reconstruction (M group); mastectomy with implant-based reconstruction including ADM (I group) and mastectomy with flap-based reconstruction with pedicle and/or free flaps (F group). Patients who received neoadjuvant chemotherapy and those whose adjuvant therapy was delayed for social reasons or patient preference were excluded.
The primary end point for the study was the time interval from the final definitive surgical procedure for breast cancer to the first day of adjuvant therapy. From this we plan to assess for any difference between the three groups in patients who started adjuvant therapy before 31 days and after 31 days of breast surgery. We also assessed for a difference in the mean interval from surgery to the start of adjuvant therapy between three different groups.
Surgical site complications recorded included infection, seroma, haematoma, skin flap necrosis and implant specific complications. Return to surgery and systemic complications were separately recorded. Complications were classified as minor if they were grade 1, 2 or 3a on the Clavien-Dindo system [19] and as major if the complications were grade 3b to 5.
From the results of Hamahata et al. [20] a standard deviation of 13 days for the delay to adjuvant chemotherapy between the two groups (IBR and non-IBR) is assumed. Thus, to compare two group means a minimum important difference of half a standard deviation is 6.5 days, and the minimum sample size required for a two-sample, two-sided t-test at the 5% significance level to give a power of 80% is 64 patients in each group.
Consecutive female patients, undergoing a total mastectomy and recommended to have adjuvant chemotherapy or radiotherapy were identified from the multidisciplinary database for a period from March 2014 to August 2014 during which the 64 patients required in the M group were identified. In order to obtain the sample size of 64 patients in I and F group, the database was sequentially reviewed over the previous years.
For data description, categorical variables are presented as counts and analysed using Fisher’s Exact test, and continuous variables are shown as mean, median, standard deviation, inter-quartile range, and range. For the statistical inference, the means of the interval from surgery to adjuvant chemotherapy for the three groups are compared using analysis of variance using a permutation F-test. Analyses have been performed using the computer program R [21]. Model fitted means have been obtained using function effect from R package effects [22,23]. The permutation test for one-way analysis of variance has been done using function aovp from R package lmPerm [24]. Bootstrap estimates and confidence limits have been obtained using functions boot and boot.ci from R package boot [25,26]. The main purpose of the statistical analysis is to estimate the “group effects”, that is the difference between the procedure means.
One hundred and ninety-two (192) patients were included in the study (64 in each of the three groups). There are statistically significant differences between the three groups with respect to age, smoking and previous breast surgery (p < 0.05 for these variables). The groups were comparable with respect to BMI, ASA grade, previous radiotherapy and contralateral surgery as shown in table 1.
Table 1. Patient characteristics
Characteristics |
Factors |
M (n = 64) |
I (n = 64) |
F (n = 64) |
Fischer Exact Test using
10000 simulations |
Age |
Mean (Range) |
59.34
(33-86) |
50.2
(27-74) |
50.61 (32-83) |
ANOVA - significant |
BMI |
Mean (Range) |
27.1
(17-33.8) |
25.9
(18-37) |
26.1
(19-34) |
Permutation test p 0.476 |
Smoking |
Yes |
16 |
4 |
2 |
p 0.0004 |
No |
45 |
52 |
49 |
Unrecorded |
3 |
8 |
13 |
Previous Surgery |
Yes |
4 |
4 |
15 |
p 0.0002 |
No |
60 |
59 |
43 |
Unrecorded |
0 |
1 |
6 |
Previous Radiotherapy |
Yes |
3 |
3 |
4 |
p 0.070 |
No |
61 |
60 |
54 |
Unrecorded |
0 |
1 |
6 |
ASA Grade |
1 |
28 |
31 |
25 |
p 0.059 |
2 |
31 |
27 |
32 |
3 |
5 |
1 |
1 |
Unrecorded |
0 |
5 |
6 |
Contralateral Surgery |
Yes |
13 |
12 |
8 |
p 0.485 |
No |
51 |
52 |
56 |
There are statistically significant differences between the three groups with respect to nodal status (p = 0.0007). The groups were comparable with respect to type of breast cancer, tumour size, grade, ER, Her 2 status and LVI as shown in table 2.
Table 2. Tumour Characteristics
Characteristics |
Factors |
M (n = 64) |
I (n = 64) |
F (n = 64) |
Fischer Exact Test using
10000 simulations |
Types |
Ductal |
45 |
53 |
56 |
p 0.080 |
Lobular |
14 |
6 |
7 |
Others |
5 |
5 |
1 |
Tumour stage |
T0 |
0 |
0 |
2 |
p 0.054 |
T1 |
9 |
10 |
11 |
T2 |
40 |
45 |
36 |
T3 |
9 |
10 |
11 |
T4 |
0 |
1 |
14 |
Unrecorded |
6 |
3 |
0 |
Nodal stage |
N0 |
11 |
29 |
22 |
p 0.0007 |
N1 |
32 |
28 |
35 |
N2 |
12 |
2 |
6 |
N3 |
1 |
0 |
0 |
Unrecorded |
8 |
5 |
1 |
Grade |
1 |
2 |
3 |
1 |
p 0.054 |
2 |
30 |
17 |
27 |
3 |
26 |
42 |
34 |
Unrecorded |
6 |
2 |
2 |
ER status |
Positive |
48 |
51 |
54 |
p 0.671 |
Negative |
13 |
12 |
9 |
Unrecorded |
3 |
1 |
1 |
Her 2 status |
Positive |
20 |
19 |
13 |
p 0.598 |
Negative |
41 |
42 |
49 |
Unrecorded |
3 |
3 |
2 |
LVI |
Yes |
33 |
27 |
17 |
p 0.053 |
No |
24 |
26 |
32 |
Unrecorded |
7 |
11 |
15 |
Six patients in the I group and one patient in the F group but no patient in the M group started adjuvant therapy within 31 days. Since only 7 patients out of 192 started their adjuvant therapy within 31 days, no clinically significant conclusions can be drawn from its analysis. The mean interval to the start of adjuvant therapy after surgery was 63.2 (33–202) days for the M group, 52.82 (26–136) days for the I group and 50.61 (29–89) days for the F group (Figure 1). Starting of adjuvant radiotherapy (when given without chemotherapy) was longer after surgery in all three groups compared to the duration to the starting of adjuvant chemotherapy. P-value for association between time to adjuvant therapy category and procedure is 0.019 indicating that the variation among the means for the three procedures are statistically significant.
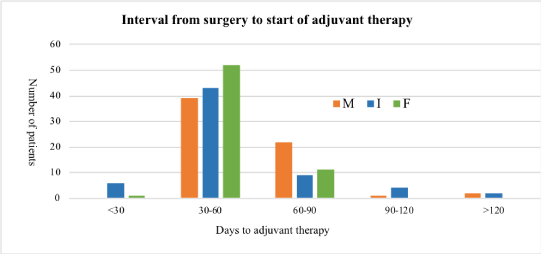
Figure 1. Interval from surgery to start of adjuvant therapy in the M, I and F groups.
Figure 2 shows the differences between the procedure means. The nominal 95% confidence interval has been adjusted by the Dunn-Sidak method [27] to allow for the multiplicity of comparisons which requires a 98.3% confidence level.
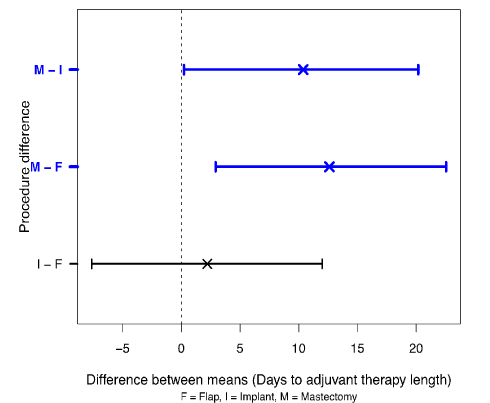
Figure 2. Differences between mean of days to adjuvant therapy for procedure and bootstrap 98.3% confidence limits.
The confidence limits for (M – F) and (M – I) do not enclose zero and so these differences between the means would usually be regarded as statistically significant.
Complications and its impact on delivery of adjuvant therapy
Patients in the I group had the highest complication rate of 28.1% compared to those in the F group (18.8%) and M group (12.5%) which were statistically significant (p = 0.005). The association between complications and the different groups are depicted in table 3. Infective complications were the commonest reason which resulted in a delay to the start of adjuvant therapy. The probability p = 0.036 as shown in table 3 indicates that the variation among the means for complications is statistically significant.
Table 3. Complications by procedure
|
F |
I |
M |
Total |
Count |
% |
Count |
% |
Count |
% |
Count |
% |
Major |
11 |
17.2 |
11 |
17.2 |
2 |
3.1 |
24 |
12.5 |
Minor |
1 |
1.6 |
7 |
10.9 |
6 |
9.4 |
14 |
7.3 |
None |
52 |
81.2 |
46 |
71.9 |
56 |
87.5 |
154 |
80.2 |
Overall |
64 |
100.0 |
64 |
100.0 |
64 |
100.0 |
192 |
100.0 |
Fisher's Exact test P-value for association between Complications and Procedure is 0.005 using 10000 simulations.
Service delays
Patients from the M, I and the F groups were discussed in the multidisciplinary team briefing and decisions made to commence adjuvant therapy in 16.69, 18.13 and 12.7 days respectively and attended an oncology appointment after additional 16.13, 9.08 and 10.1 days but received adjuvant treatment after another 29.18, 25.61 and 27.81 days. This shows the initiation of treatment was significantly delayed in all groups from the time of decision-making irrespective of complications or oncology appointment.
It is well established that breast reconstruction after mastectomy improves the quality of life and in fact the improved psychological outlook is more pronounced in IBR compared with delayed reconstruction [28]. While IBR has been suggested to be oncologically safe, there remains a concern that its complication rates may be higher than mastectomy without reconstruction [29-31] and this may unduly delay the initiation of systemic chemotherapy or lead to its omission altogether [7,32,33]. This study looked at the effects of modern methods of breast reconstruction like DIEP flap and ADM based implant reconstruction on delivery of adjuvant chemotherapy and radiotherapy.
In this study, the patients in the M group were found to reflect the patient characteristics in the series by Zhong et al. [34] in which patients having mastectomy alone were older (median age 51 vs. 45 years, p < 0.0001) and more likely to be smokers (14% vs. 5%, p = 0.007). This may represent a selection bias where younger fitter patients request and accept immediate reconstruction more readily.
The patients undergoing IBR were significantly more node negative and there were also more T3 and higher-grade tumours in the flap group but were not statistically significant. In the review by Chang RJ et al. [35], there were more women with stage I and II tumours in the IBR group compared to the group who received mastectomy alone (72.0% versus 57.5%, p = 0.034) and also had fewer positive nodes and more grade I and II tumours (42.4% versus 63.6%, p = 0.006).
Only 7 patients out of 192 (6 in the implant group and one patient in the flap group) met the NICE target of 31 days to the start of adjuvant therapy after final surgery. This is mainly a reflection of the service delays. Most published series show a time to start of adjuvant therapy of more than 31 days. In the 2012 NHS Breast Screening Program (NHSBSP)/Association of Breast Surgery (ABS) national audit of adjuvant therapy for screen-detected breast cancers diagnosed in 2009/10, in the whole of UK, the median time from final surgery to radiotherapy was 60 days (inter-quartile range 48-74 days) [36]. Fewer than 50% of women received radiotherapy within eight weeks of their final surgery [37].
In our results, patients who had mastectomy without reconstruction had a greater delay to start adjuvant therapy compared to reconstructed patients. This is also highlighted in the series by Allweis [38] with 52.7 (range 1 to 215) days for mastectomy alone versus 40.6 (range 14 to 131) for patients with reconstruction. This observation may be explained by the finding that mastectomy patients were older, more likely to be smokers and less fit prolonging the recovery from surgery.
In this study, we found an overall complication rate of 12.5% for mastectomy patients, 28.1% for implant patients and 18.8% for flap patients. This reflects the trend shown in other series like Mortenson et al. [39] where complications in patients who underwent immediate breast reconstruction compared with those who did not was 17/76 [22.3%] vs 6/72 [8.3%]; p = 0.02. In the review by Zhong et al. [34] patients undergoing mastectomy alone had a 3.7% major complication compared to 15.5% in the IBR group (p < 0.0001). In the series by Shikhman et al. [40], there was a 15.3% complication rate in 98 non-IBR patients compared to a 24.2% complication in 66 IBR patients. Those with complications had a statistically significant delay to initiation of chemotherapy (42.5 days vs 60.6 days, p = 0.013). According to our results, the higher complication rate in our reconstruction group did not lead to an overall delay in starting adjuvant therapy. This may be because many of the complications after reconstruction like bleeding and skin or flap necrosis are dealt with by early further definitive surgical procedures without delaying recovery significantly.
Apart from the patient, tumour and surgical factors discussed above, service delivery capacity will also affect the time to delivery of adjuvant therapy. These include the time required for pathologic assessment of the tumour, referral wait time to see an oncologist and capacity to deliver chemotherapy or radiotherapy. This study was conducted in a single region of the United Kingdom which is likely to provide similar capacity to deliver adjuvant therapy thereby limiting this effect on our results. Alderman AK [41] found that in addition to age, other clinical and socio-demographic characteristics place patients at increased risk for delayed chemotherapy. Taylor et al. [42] found that the reasons for late chemotherapy initiation in the non-reconstructed group included the need for pathology review and social reasons like patient holidays.
Treatment pathway management can minimise the total time from final surgery to the start of adjuvant therapy [37]. Multi-disciplinary teams should plan adjuvant therapy well ahead to try to ensure that women have their treatment at the earliest appropriate time [37]. Seeing patients in a multidisciplinary breast clinic with surgeons, oncologists and breast care nurses as soon as possible can reduce some of the service delays. There are considerable regional differences in service provision for radiotherapy in the UK. From the 2009/10 breast screening data, the median number of days varied from 53 days in North West, to 69 days in South East Coast [36]. Our study was conducted as a multicentre audit which increases its applicability to other NHS trusts in the UK.
Our data has been collected as a retrospective study and has limitations compared to a prospective trial. There might be unaccounted factors that could be associated with patient characteristics, treatment choice and timing of delivery of adjuvant therapy resulting in bias. All free flap reconstructions were performed at one centre which has one of the highest volumes of free flap breast reconstruction in Europe and the results may not translate to small volume centers.
In conclusion, this study shows that the majority of patients undergoing mastectomy regardless of IBR will have a delay to the start of adjuvant therapy beyond 31 days. Patients who underwent mastectomy alone had a statistically significant delay to the start of adjuvant therapy compared to the implant and free flap groups, but this group of patients were older and more likely to be smokers. The clinical significance of this delay is not clear. The incidence of postoperative complications was significantly higher after IBR than mastectomy alone. Patients who had post-operative complications had a delayed start of adjuvant therapy compared to patients who made an uncomplicated recovery.
The authors would like to acknowledge Miles-Dua S, Rothnie ND, Gray EA, Lawrence J, Aitken J and Parker M for their contribution to this article
- Cancer Research UK, Cancer Statistics Reports for the UK. Available at: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer 2015 [Accessed 03 July 2018].
- Dean C, Chetty U, Forrest AP (1983) Effects of immediate breast reconstruction on psychosocial morbidity after mastectomy. Lancet 1: 459-462. [Crossref]
- Rosenqvist S, Sandelin K, Wickman M (1996) Patients’ psychological and cosmetic experience after immediate breast reconstruction. Eur J Surg Oncol 22: 262-266. [Crossref]
- D'Souza N, Darmanin G, Fedorowicz Z (2011) Immediate versus delayed reconstruction following surgery for breast cancer. Cochrane Database Syst Rev 7: CD008674. [Crossref]
- Atisha D, Alderman AK, Lowery JC, Kuhn LE, Davis J, et al. (2008) Prospective analysis of long-term psychosocial outcomes in breast reconstruction: 2-year postoperative results from the Michigan Breast Reconstruction Outcomes Study. Ann Surg 247: 1019 -1028. [Crossref]
- Drucker-Zertuche M, Robles-Vidal C (2007) A 7-year experience with immediate breast reconstruction after skin sparing mastectomy for cancer. Eur J Surg Oncol 33:140-146. [Crossref]
- Rainsbury RM (2006) Skin-sparing mastectomy. Br J Surg 93: 276-281. [Crossref]
- Giacalone PL, Rathat G, Daures JP, Benos P, Azria D, et al. (2010) New concept for immediate breast reconstruction for invasive cancers: feasibility, oncological safety and aesthetic outcome of post-neoadjuvant therapy immediate breast reconstruction versus delayed breast reconstruction: a prospective pilot study. Breast Cancer Res Treat 122: 439- 451. [Crossref]
- Langstein HN, Cheng MH, Singletary SE, Robb GL, Hoy E, et al. (2003) Breast cancer recurrence after immediate reconstruction: patterns and significance. Plast Reconstr Surg 111: 712-720. [Crossref]
- Newman LA, Kuerer HM, Hunt KK, Ames FC, Ross MI, et al. (1999) Feasibility of immediate breast reconstruction for locally advanced breast cancer. Ann Surg Oncol 6: 671-675. [Crossref]
- Oncoplastic Breast Reconstruction. Guidelines for Best Practice. (2012) Available at: http://www.bapras.org.uk/docs/default-source/commissioning-and-policy/final-oncoplastic-guidelines---healthcare-professionals. (last accessed 03 July 2018)
- Vaughan A, Dietz JR, Aft R, Gillanders WE, Eberlein TJ, et al. (2007) Patterns of local breast cancer recurrence after skin-sparing mastectomy and immediate breast reconstruction. Am J Surg 194: 438-443. [Crossref]
- Patani N, Devalia H, Anderson A, Mokbel K (2008) Oncological safety and patient satisfaction with skin-sparing mastectomy and immediate breast reconstruction. Surg Oncol 17: 97-105. [Crossref]
- National Mastectomy and breast reconstruction audit. (2011) Available at: https://www.rcseng.ac.uk/-/.../national-mastectomy-and-breast-reconstruction-audit. (last accessed 03 July 2018).
- Early Breast Cancer Trialists’ Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365: 687-717. [Crossref]
- EBCTCG (Early Breast Cancer Trialists' Collaborative Group), McGale P, Taylor C, Correa C, Cutter D, et al. (2014) Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 383: 2127-2135. [Crossref]
- National Institute for Clinical Excellence. (2017) CG80- Early and locally advanced breast cancer: Diagnosis and treatment. https://www.nice.org.uk/guidance/cg80. (last accessed 03 July 2018)
- Desch CE, McNiff KK, Schneider EC, Schrag D, McClure J, et al. (2008) American Society of Clinical Oncology/National Comprehensive Cancer Network quality measures. J Clin Oncol 26: 3631-3637. [Crossref]
- Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240: 205-213. [Crossref]
- Hamahata A, Kubo K, Takei H, Saitou T, Hayashi Y, et al. (2015) Impact of immediate breast reconstruction on postoperative adjuvant chemotherapy: a single centre study. Breast Cancer 22: 287-291. [Crossref]
- Dean CB, Nielsen JD (2007) Generalized linear mixed models: a review and some extensions. Lifetime Data Anal 13: 497-512. [Crossref]
- Fox J (2003) Effect Displays in R for Generalised Linear Models. J Stat Softw 8: 1-27.
- Fox J, Hong J (2009) Effect Displays in R for Multinomial and Proportional-Odds Logit Models: Extensions to the effects Package. J Stat Softw 32: 1-24.
- Bob Wheeler (2010) lmPerm: Permutation tests for linear models. R package version 1.1-2. Available at: http://CRAN.R-project.org/package=lmPerm
- Canty A, Ripley B (2014) boot: Bootstrap R (S-Plus) Functions. R package version 1.3-13.
- Davison AC, Hinkley DV (1997) Bootstrap Methods and Their Applications. Cambridge University Press, Cambridge. ISBN 0-521-57391-2
- Dunn OJ (1961) Multiple Comparisons Among Means. J Am Stat Assoc 56: 52-64.
- Al-Ghazal SK, Sully L, Fallowfield L, Blamey RW (2000) The psychological impact of immediate rather than delayed breast reconstruction. Eur J Surg Oncol 26: 17 - 19.
- Yeh KA, Lyle G, Wei JP, Sherry R (1998) Immediate breast reconstruction in breast cancer: morbidity and outcome. Am Surg 64: 1195-1199. [Crossref]
- Alderman AK, Wilkins EG, Kim HM, Lowery JC (2002) Complications in post mastectomy breast reconstruction: two-year results of the Michigan breast reconstruction outcome study. Plast Reconstr Surg 109: 2265 - 2274. [Crossref]
- Ringberg A, Tengrup I, Aspegren K, Palmer B (1999) Immediate breast reconstruction after mastectomy for cancer. Eur J Surg Oncol 25: 470-476. [Crossref]
- Caffo O, Cazzolli D, Scalet A, Zani B, Ambrosini G, et al. (2000) Concurrent adjuvant chemotherapy and immediate breast reconstruction with skin expanders after mastectomy for breast cancer. Breast Cancer Res Treat 60: 267-275. [Crossref]
- Vandeweyer E, Deraemaecker R, Nogaret JM, Hertens D (2003) Immediate breast reconstruction with implants and adjuvant chemotherapy: a good option? Acta Chir Belg 103: 98-101. [Crossref]
- Zhong T, Hofer SO, McCready DR, Jacks LM, Cook FE, et al. (2012) A comparison of surgical complications between immediate breast reconstruction and mastectomy: the impact on delivery of chemotherapy - an analysis of 391 procedures. Ann Surg Oncol 19: 560-566. [Crossref]
- Chang RJ1, Kirkpatrick K, De Boer RH, Bruce Mann G (2013) Does immediate breast reconstruction compromise the delivery of adjuvant chemotherapy? Breast 22: 64-69. [Crossref]
- NHS breast screening (BSP) programme. Available at: http://www.cancerscreening.nhs.uk/breastscreen/publications/baso2009-2010.pdf. [Accessed 21 July 2018].
- Time from final surgery to radiotherapy for screen-detected breast cancers. Avaiable at: http://www.ncin.org.uk/publications/data_briefings/time_from_final_surgery_to_radiotherapy_for_screen_detected_breast_cancers. [Accessed 21 July 2018].
- Allweis TM, Boisvert ME, Otero SE, Perry DJ, Dubin NH, et al. (2002) Immediate reconstruction after mastectomy for breast cancer does not prolong the time to starting adjuvant chemotherapy. Am J Surg 183:”218-221. [Crossref]
- Mortenson MM, Schneider PD, Khatri VP, Stevenson TR, Whetzel TP, et al. (2004) Immediate breast reconstruction after mastectomy increases wound complications: however, initiation of adjuvant chemotherapy is not delayed. Arch Surg 139: 988-991. [Crossref]
- Shikhman L, Dinh K, O'Connor A, Larkin AC (2014) Time to treatment: Influence of immediate breast reconstruction on postoperative chemotherapy. Ann Surg Oncol 21: 110.
- Alderman AK, Collins ED, Schott A, Hughes ME, Ottesen RA, et al. (2010) The impact of breast reconstruction on the delivery of chemotherapy. Cancer 116: 1791-1800. [Crossref]
- Taylor CW, Kumar S (2005) The effect of immediate breast reconstruction on adjuvant chemotherapy. Breast 14: 18 - 21. [Crossref]


