Case report
A persistent left-superior vena cava (PLSVC) is an uncommon identification with a form of 0.3% to 0.5% of persons in the common population [1-5]. Nevertheless, it is the most common thoracic venous anomaly [6-10]. Typically, the left superior vena cava fades post embryological developement. The identification can be missed by the manifestation of a standard right superior vena cava. This subject did not have an ordinary or rest of a right superior vena cava. Furthermost of the individuals do not present the symptoms, and the presence of the persistent left superior vena cava is by the way found during or after insertion of a central venous catheter (CVC) or pacemaker electrodes. The correct report of a PLSVC and lack of a right superior vena cava has significant clinical repercussions in definite circumstances, such as oncological therapy, totally implantable vessels catheters, hemodynamic checking in intenive care unit (ICU) or the correct location of pacemakers [1-10]. The further clinical relevance of the described anomaly could be due to common tachyarrhythmia and conduction disturbances [11-15]. The PLSVC usually descends vertically, anterior, and to the left of the aortic arch and main pulmonary artery. It runs adjacent to the left atrium (LA) before turning medially, piercing the pericardium to run in the posterior atrioventricular (AV) groove [16]. In about 90% of cases, it drains into the coronary sinus (CS); alternative sites include the inferior vena cava, hepatic vein, and LA. The entry into LA is invariably associated with an atrial septal defect ASD [17,18].
In this case, we describe a female patient, 63 years old, with hypertension and dilated cardiomyopathy, without coronary artery disease. She was recovered from sudden cardiac death, with previous events of syncope, dyspnea on habitual exertion, and pre-syncope that began 6 months ago. She also was in use of acetylsalicylic acid 100 daily, carvedilol 25 twice a day, digoxin 0.25 mg per day, furosemide 40 mg daily, atorvastatin 40 mg daily, spironolactone 50 mg daily. The basal eletrocardiogram (ECG) presented sinus rhythm and QRS complex duration 170 ms. The 24-hour Holter monitoring showed sinus rhythm, with minimum – average – maximum heart rate (HR) of 39, 56 and 93 bpm, respectively, as well as, 6737 polymorphic ventricular ectopic beats and 5 episodes of non-sustained ventricular tachycardya, being the highest composed 16 beats at 180 bpm. The transthoracic echocardiogram showed: LA 4,3 cm, LVED 6,3 cm, LVES 5,8 cm, LVEF 17,2%, left ventricular mass index 139,3 g/m2, andd diffuse hypokinesia of the left ventricle. The coronary angiography did not present any new obstruction.
The patient was submitted to general anesthesia by an anesthesiologist, and 2 g of cefazolin was administered intravenously. During the surery, a persistent left superior vena cava (PLSVC) was perceived. The left venography revealed a lack of contrast filling in an innominate vein (IV) and a quadripolar diagnostic catheter of electrophysiology within the CS introduced into the right femoral vein, as shown in Figure 1A and B, respectively. Post several efforts we succeeded in placing the “double-coil” shock lead into the right cephalic vein by dissection, then through the IV and right superior vena cava into the apex of the right ventricle (Figure 1C). After a double puncture of the right axillar vein, by one of them, a long sheath was positioned faced to the CS ostium, and the contrast was injected non-selectively filling this structure, showing how big was the CS in this case (Figure 1D). Subsequently, the angiography of coronary arteries was performed, the quadripolar diagnostic catheter was moved to the right outflow tract, and the long sheath was fully inserted into the CS (Figure 1E), then it was pulled back and selective injection of contrast was done with the aid of a Swan-Ganz catheter (Figure 1F). The guide wire was positioned inside the lower-posterior vein, and the quadripolar diagnostic catheter was pulled back to be removed (Figure 1G). A bipolar CS lead was placed into the vein selected, the “double-coil” shock lead was maintained in the same position, the atrial lead was inserted via the other right axillar puncture being actively fixed in the upper right atrium wall, the leads were fixed in the right pectoral muscle and connected to an implantable cardioverter defibrillator with cardiac resynchronization therapy (ICD-CRT), as shown in Figure 1H.
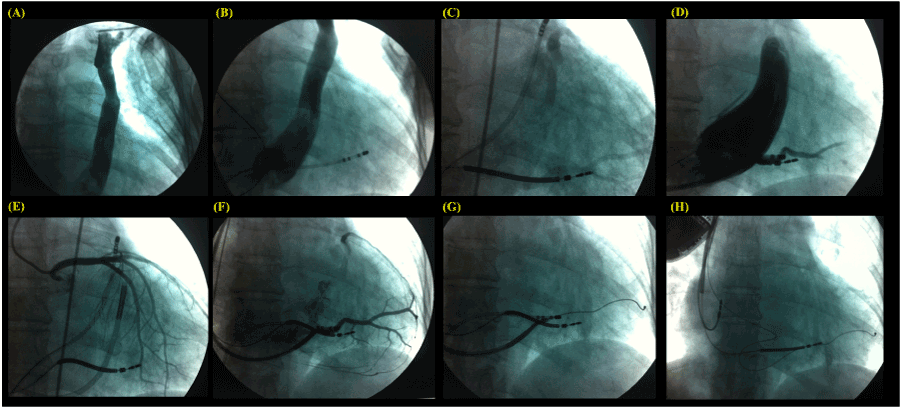
Figure 1. The left venography revealed a lack of contrast filling in an innominate vein (IV) and a quadripolar diagnostic catheter of electrophysiology within the coronary sinus (CS), A and B respectively. The “double-coil” shock lead was positioned in the apex of the right ventricle (C). Long sheath positioned faced to the CS ostium, and the contrast was injected non-selectively filling this structure (D). Subsequently, the angiography of coronary arteries was performed (E), and selective injection of contrast was done with the aid of a Swan-Ganz catheter (F). The guide wire was positioned inside the lower-posterior vein (G). A bipolar CS lead was placed into the vessel selected, the “double-coil” shock lead was maintained in the same position, the atrial lead was actively fixed in the upper right atrium wall, and connected to the implantable cardioverter defibrillator with cardiac resynchronization therapy (ICD-CRT) device (H).
The following devices parameters were measured at the end of the procedure:
Leads |
Sense (mV) |
Impedance (Ω) |
Threshold (V) @ 0.5ms |
Atrial |
P = 2.8 |
502 |
0.50 |
Right ventricular |
Right R = 16.2 |
624 |
0.50 |
Left ventricular |
Left R = 19.0 |
711 |
0.75 |
The biventricular pace measured was 129 ms. After 48 hours the patient was discharged, using the same medications; no AF episodes were recorded by the ICD+CRT, and this one presented normal parameters. Until the present time of follow up (1 month), the patient presented improvement of the symptoms, without arrhythmic events, and keeping the same parameters of the moment of the implant
Acknowledgements
We would like to Pacem2021 Copyright OAT. All rights reserv
References
- Sarodia BD, Stoller JK (2000) Persistent left superior vena cava: case report and literature review. Respir Care 45: 411-416. [Crossref]
- Povoski SP, Khabiri H (2011) Persistent left superior vena cava: a review of the literature, clinical implications, and relevance of alterations in thoracic central venous anatomy as pertaining to the general principles of central venous access device placement and venography in cancer patients. World J Surg Oncol 9: 173.
- Parreira LF, Lucas CC, Gil CC, Barata JD (2009) Catheterization of a persistent left superior vena cava. J Vasc Access 10: 214-215. [Crossref]
- Granata A, Andrulli S, Fiorini F, Logias F, Figuera M, et al. (2009) Persistent left superior vena cava: what the interventional nephrologist needs to know. J Vasc Access 10: 207–211. [Crossref]
- Goyal SK, Punnam SR, Verma G, Ruberg FL (2008) Persistent left superior vena cava: a case report and review of the literature. Cardiovasc Ultrasound 6: 50.
- Wissner E, Tilz R, Konstantinidou M, Metzner A, Schmidt B, et al. (2010) Catheter ablation of atrial fibrillation in patients with persistent left superior vena cava is associated with major intraprocedural complications. Heart Rhythm 7: 1755–1760. [Crossref]
- Jolly N, Dhar G, Anderson A (2010) Stenting for persistent left superior vena cava syndrome. J Invasive Cardiol 22: 92-93. [Crossref]
- Lee MS, Pande RL, Rao B, Landzberg MJ, Kwong RY (2011) Cerebral abscess due to persistent left superior vena cava draining into the left atrium. Circulation 124: 2362–2364. [Crossref]
- Gonzalez-Juanatey C, Testa A, Vidan J, Izquierdo R, Garcia-Castelo A, et al. (2004) Persistent left superior vena cava draining into the coronary sinus: report of 10 cases and literature review. Clin Cardiol 27: 515–518. [Crossref]
- Tsutsui K, Ajiki K, Fujiu K, Imai Y, Hayami N, et al. (2010) Successful catheter ablation of atrial tachycardia and atrial fibrillation in persistent left superior vena cava. Int Heart J 51: 72-74. [Crossref]
- Motta-Leal-Filho JM, Motta CA, Pilan BF, Affonso BB (2014) Persistent left superior vena cava. Ann Thorac Surg 97: 1453. [Crossref]
- Giebel J, Fanghänel J, Hauser S, Paul I (2000) A case of a persistent left vena cava superior with atresia of the right atrial ostium of the coronary sinus. Ann Anat 182: 191-194. [Crossref]
- Schummer W, Schummer C, Fröber R (2003) Persistent left superior vena cava and central venous catheter position: clinical impact illustrated by four cases. Surg Radiol Anat 25:315–321. [Crossref]
- Schiffmann L, Kruschewski M, Wacker F, Buhr HJ (2003) Persistent left superior vena cava: a reason for pseudodisplacement of a port catheter. Surg Radiol Anat 25: 70-72. [Crossref]
- Peltier J, Destrieux C, Desme J, Renard C, Remond A, et al. (2006) The persistent left superior vena cava: anatomical study, pathogenesis and clinical considerations. Surg Radiol Anat 28: 206-210. [Crossref]
- Lenox CC, Hashida Y, Anderson RH, Hubbard JD (1985) Conduction tissue anomalies in absence of the right superior caval vein. Int J Cardiol 8: 251-260. [Crossref]
- Postema PG, Rammeloo LA, van Litsenburg R, Rothuis EG, Hruda J (2008) Left superior vena cava in pediatric cardiology associated with extra-cardiac anomalies. Int J Cardiol 123: 302-306. [Crossref]
- Uçar O, Paşaoğlu L, Ciçekçioğlu H, Vural M, Kocaoğlu I, et al. (2010) Persistent left superior vena cava with absent right superior vena cava: a case report and review of the literature. Cardiovasc J Afr 21: 164-166. [Crossref]

