Aging occurs in response to many triggers, including but not limited to cellular senescence, telomere attrition, DNA damage, organelle stress, oncogene activation and stem cell dysfunction [1]. Among these, the aged stem cell per se can exhibit various aging hallmarks, such as increased DNA damage, dysregulated mitochondrial function, and less efficient protein recycling. Aged stem cell is a prime factor that undermines organismal homeostasis, which can facilitate numerous pathologies including cancer, neuropathy, sarcopenia and immunopathy [2].
Tissue function is subject to daily fluctuations. Circadian rhythm is a fundamental part of biology, as most tissues have the internal circadian clock to orchestrate rhythmic physiological adaptation to the diurnal- nocturnal cycle [3]. The mammalian master clock locates in the suprechiasmatic nucleus (SCN) and coordinates central nervous system (CNS) and peripheral tissue oscillators [4]. Increasing evidence suggests that circadian rhythms are dysregulated along the path of aging. Reciprocally, the disrupted circadian clock can exacerbate a broad scope of diseases, ranging from cancer to neurodegenerative diseases [5].
Adult stem cell is the mainstay for regeneration among various tissues, however, it’s function is also relevantly impaired during aging. The aged stem cell can be the consequence of either holistic natural aging or dealing with various stresses confined within a particular tissue [6]. However, whether the dysregulated circadian rhythm and aged stem cell are coupled to any cause-and-effect relationship remains a subject of largely unknown.
There has been a number of recent exciting studies documenting how aged stem cell affects the molecular clock in several tissues. The adult epidermal stem cell and skeletal muscle stem cell reprogram their rhythmic transcriptome and lose their rhythmic expression of homeostasis genes upon aging [7]. The oscillatory rewiring results in a de novo rhythmic transcriptome predominantly associated with DNA damage and loss of basal autophagy. This age-associated reprogramming of the oscillatory transcriptome can be significantly rescued in old mice by long-term caloric restriction.
Likewise, the disrupted circadian clock can also impair stem cell function and renewal. An exemplary study showed that the Clock△19 mutant mouse display impaired stem cell function and renewal [8]. This suggests that genetically disrupted circadian clocks can drive dysfunction of tissue-specific stem cells.
A plausible hypothesis to disentangle the intertwined relationship between aged stem cell and dysregulated circadian clock is that the disrupted pacemaker serves as an inherent aging hallmark in aged stem cell in the first place, which signals to the molecular network within the aged stem cell, that in turn, reinforces the aging roadmap. The missed link in this proposition is how could the rewired circadian clock get sensed to the genetic hub of aged stem cell? Based on the recent studies, one potential crosstalk could be the inflammation.
A body of recent studies have demonstrated the circadian clock regulates inflammatory and oxidative stress responses. For example, either lesion of the SCN or the light-induced rhythmic dispersion can aggravate the release of cytokines TNFa and IL-6 [9,10]. In mouse models of Bmal1 KO or Clock/Npas2 KO, the mice develop aging-associated pathologies as well as the increased chronic inflammation [11,12]. In monkeys, Bmal1 KO induces overactivated immune response concomitant with increased inflammation and depression-like symptoms [13].
The increased inflammatory cytokine secretion has been widely investigated in the context of aging. Of note, an inflammatory microenvironment is a favorable factor for DNA damage and tumorigenesis [14]. DNA damage and markers of DNA damage response (DDR) can be observed in various stem cell types during natural and pathological aging [15,16]. The DDR cascade in aged stem cells can lead to permanent cell cycle arrest with features of cellular differentiation and senescence, in other words, the depletion of stem cell pool.
To integrate the following elements, namely, the dysregulated circadian rhythm, amplified inflammation, reinforced DDR and accelerated aging of stem cell, we can possibly draw an aging roadmap in stem cell delineating the potential priming role of circadian rhythm and the crosstalk effect of inflammation (Figure 1).
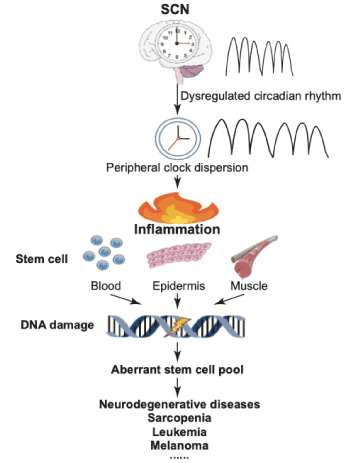
Figure 1. A scheme for postulated crosstalk among circadian clock, inflammation, stem cell and diseases
Of note, more meticulous investigations are required to further validate this model. For example, how would the central and peripheral circadian system synchronize during aging, how could immune cells respond to peripheral clock dispersion and potentiate inflammation, etc?
Nevertheless, the intriguing relationship between dysregulated circadian rhythm and aged stem cell has been recently entered the spotlight for its great implication in aging and disease study, along with a growing body of evidence documenting the noticeable involvement of inflammation, which deserves more demonstration for its potential crosstalk effect.
This work was supported by the Natural Science Foundation of China 81900107 (Y.T) and Shanghai Sailing Program 19YF1429500 (Y.T).
- Lopez-Otin C, Blasco MA, Partridge L, SerranoM, Kroemer G (2013) The hallmarks of aging. Cell 153: 1194-1217. [Crossref]
- Ahmed AS, Sheng MH, Wasnik S, Baylink DJ, Lau KW, et al. (2017) Effect of aging on stem cells. World J Exp Med 7: 1-10. [Crossref]
- Patke A, Young MW, Axelrod S (2019) Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol 21: 67-84. [Crossref]
- Hastings MH,Maywood ES, Brancaccio M (2018) Generation of circadian rhythms in the suprachiasmatic nucleus. Nat Rev Neurosci 19: 453-469. [Crossref]
- Yu EA, Weaver DR (2011) Disrupting the circadian clock: gene-specific effects on aging, cancer, and other phenotypes. Aging (Albany NY) 3: 479-493.
- Van Zant G, Liang Y (2003) The role of stem cells in aging. Exp Hematol 31: 659-672.
- Solanas G (2017) Aged stem cells reprogram their daily rhythmic functions to adapt to stress. Cell 170: 678-692. [Crossref]
- Yang N (2017) Cellular mechano-environment regulates the mammary circadian clock. Nat Commun 8: 14287.
- Guerrero-Vargas NN (2014) Reciprocal interaction between the suprachiasmatic nucleus and the immune system tunes down the inflammatory response to lipopolysaccharide. J Neuroimmunol 273: 22-30. [Crossref]
- Adams KL, Castanon-Cervantes O, Evans JA, Davidson AJ (2013) Environmental circadian disruption elevates the IL-6 response to lipopolysaccharide in blood. J Biol Rhythms 28: 272-277. [Crossref]
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP, et al. (2006) Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev 20: 1868-1873. [Crossref]
- Musiek ES (2013) Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest 123: 5389-5400.
- Haque SN, Booreddy SR, Welsh DK (2019) Effects of BMAL1 manipulation on the brain's master circadian clock and behavior. Yale J Biol Med 92: 251-258. [Crossref]
- Rodier F (2009) Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol 11: 973-979. [Crossref]
- Rossi DJ (2007) Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 447: 725-729. [Crossref]
- McNeely T, Leone M, Yanai H, Beerman I (2020) DNA damage in aging, the stem cell perspective. Hum Genet 139: 309-331.

