Abstract
One of the pillars in the emergency response to the 2014-2016 Ebola virus epidemic in West Africa has been the local deployment of temporary laboratories by the international community in collaboration with local authorities. The Dutch Ministry of Foreign Affairs financed and supported the deployment of three mobile container laboratories to Sierra Leone (Freetown and Koidu) and one Liberia (Sinje). We describe the organisation of the three laboratories, the biosafety aspects, the quality control, and the performance in Ebola virus and malaria diagnostics during the period of deployment.
Keywords
ebola virus, diagnostics, west-africa, mobile laboratory, malaria
Introduction
An epidemic with Ebola virus disease (EVD) has been ongoing in West Africa in 2014-2016 affecting mainly Guinea, Liberia and Sierra Leone. As of 10 June 2016, the cumulative number of probable and confirmed cases stands at 28 616, including 11 310 deaths, making this EVD outbreak the worst in history in terms of geographic spread and number of cases and deaths reported [1]. One of the pillars in the emergency response to the EVD epidemic in the region has been the deployment of temporary (mobile) laboratories by the international community in collaboration with local authorities. Short turn-around-times (TAT) for diagnostic specimens (WHO target: within 24 hours) were an absolute necessity to control the epidemic which could solely be achieved by sufficient testing capacity with a good geographic coverage. Early 2015, 27 laboratories were installed [2]. These laboratories provided rapid testing capacity for Zaïre Ebola virus (EBOV) and malaria in support of clinical triage of (suspected) patients, determination of cause of death in hospitals and communities, and surveillance purposes.
EBOV is a Biosafety level 4 pathogen, belonging to the family Filoviridae, genus Ebolavirus. EBOV diagnostics in outbreak situations are mainly based on ((semi-) quantitative real-time) reverse-transcriptase polymerase chain reaction ((q)RT-PCR) detecting the viral RNA genome in whole blood, EDTA-plasma or body swabs from deceased persons. The EBOV genome is about 19 kb long and encodes seven proteins, designated from 5’-end to 3’-end as: NP (nucleoprotein), VP (viral protein)35, VP40, GP (glycoprotein), VP30, VP24 and L (polymerase). Multiple commercial and in-house (q)RT-PCR tests have been described for EBOV-Zaire, the majority targeting the NP, GP or L-gene [3]. Based on the published kinetics of viremia in mild and severe EVD cases from historic outbreaks, an algorithm for sampling for molecular diagnostics of EBOV has been established providing the basic rule that negative PCR results obtained on specimens < 72 hours post onset of illness are not reliable with a need to repeat the PCR on a second sample taken at least 24 hours after the initial sample and ≥ 72 hours upon illness onset [3]. As in any emerging infectious disease outbreak, the clinical sensitivity of this approach using new real-time molecular tests for the current EBOV-Zaire strain could not be determined before deployment, calling for stringent quality control and evaluation of results during deployment.
To aid in the EVD response, the Dutch Ministry of Foreign Affairs financed and supported the deployment of three mobile container laboratories. Two laboratories were placed in Sierra Leone (Freetown and Koidu), and one in Liberia (Sinje). The laboratories were set-up and their operation was coordinated by the Viroscience department of Erasmus Medical Centre, Rotterdam, the Netherlands. They were operated locally by volunteers mainly employed in various Dutch clinical and veterinary diagnostic and research centres with logistic support of the Dutch ministry of foreign affairs, the Non-Governmental Organizations “Partners in Health” (PIH) in Sierra Leone, the “International Organization for Migration” (IOM) in Liberia, and the Department For International Development (DFID), UK. Here we describe the organisation of the three Dutch mobile laboratories, the biosafety aspects, the quality control, and the performance during the period of deployment.
Materials and Methods
Set-up mobile container laboratory
The Dutch mobile laboratories were set up in either a 20 foot sea container (Freetown, Sierra Leone and Sinje, Liberia ) or a 40 foot sea container that can be mounted on a trailer (Koidu, Sierra Leone) (Hospitainer, Apeldoorn, the Netherlands [4]). The laboratory space was identical in both setups, but the 40 foot container had an adjacent office/ storage space (Figures 1). Essentially, the diagnostic equipment and flow was designed according to the clinical virology diagnostic unit of Erasmus MC, which offers a broad range of molecular diagnostic assays including those targeting high threat pathogens. The rationale for this set up was that this would provide an opportunity for transition to a longer-term sustainable molecular diagnostic laboratory [5]. The laboratory space was a biosafety-level (BSL) 2 room which was accessible through an interlock sluice where personal protective equipment (PPE) worn in the laboratory could be donned and doffed. To provide safe inactivation of samples containing high-risk pathogens in a resource low setting as encountered in West-Africa, the laboratories were equipped with a BSL3 glovebox (Plas-Labs, Michigan, USA). In addition a refrigerator, freezer, nucleic acid extraction robot (EZ1XL advanced, Qiagen, Germany) and real-time polymerase chain reaction machine (Lightcycler 96, Roche, Switzerland) were installed. The containers had their own generator and APC smart UPS. The containers were air-conditioned and equipped with dust filters and a water pump with cleaning filters. The air from the laboratory outlet is again filtered by a dust filter and a HEPA filter, ensuring maximal safety for the environment.
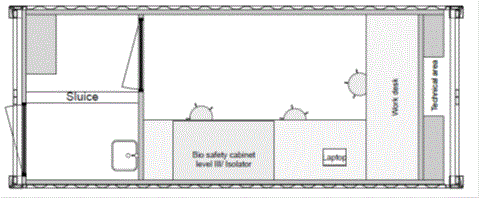
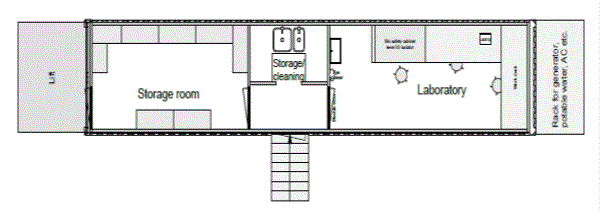
Figure 1. Lay-out of the Dutch mobile laboratories deployed to West Africa, 2015. (A). Lay-out of the 20 feet container laboratory as deployed to Sinje, Liberia and Freetown, Sierra Leone. (B). Lay-out of the 40 feet container laboratory as deployed to Kono, Sierra Leone. (copy right: Hospitainer Apeldoorn, the Netherlands).
Biosafety
In addition to the BSL3 glovebox for sample inactivation and performing malaria antigen tests, biosafety was further ensured by extensive training of personnel, working in pairs with a buddy who monitors every action, multiple decontamination steps during the work flow and the obligation to don personal protective equipment (PPE) which included scrubs, front gown, hairnet, goggles or face-shield, two pairs of gloves and clogs. This very stringent regimen, which technically was safer than necessary in a clean BSL2 laboratory with BSL3 glovebox, was decided based on discussions with Erasmus MC biosafety experts and the, at the time, ongoing discussion about the potential (in)complete inactivation of EBOV with lysis buffer with chaotropic salts/triton in mind. Special attention was given to practice PPE doffing as this is considered a high-risk procedure [6]. In case of a spill in the laboratory, a specific protocol and “spill-kit” was in place that included extra PPE with a coverall and shoe covers. All personnel had to monitor their body temperature twice a day and had to report any clinical symptoms.
Malaria antigen RDT
In Liberia and initially in Sierra Leone, the presence of Plasmodium falciparum antigens was qualitatively detected by an immuno-chromatographic lateral flow assay (ICT Malaria Casette test, ICT international, South Africa) using 5µl whole blood according to manufacturer’s instructions. Upon request by the Sierra Leonean MOH, all laboratories in Sierra Leone switched to the SD Bioline Malaria test (Standard Diagnostics, Korea) in March 2015 for a nationwide standardization.
Ebola virus real-time RT-PCR
Approximately 1.5 ml whole blood was aliquoted into a 2ml Sarstedt screw-cap tube, quick-spinned (1min 14.000g) in a mini centrifuge inside the BSL3 safety cabinet to separate plasma and cells prior to inactivation. Samples were inactivated by addition of 300 µl AVL lysis buffer (Qiagen, Hilden, Germany) to 100 µL EDTA-plasma or UTM (swabs) containing an internal process control (heat-inactivated phocine distemper virus , PDV [7]). Samples were exported out of the BSL3 cabinet after a 10 min. decontamination of the sample tubes in 1% hypochlorite solution. Total nucleic acids of the inactivated samples and controls were extracted using an external lysis virus V2 protocol using the EZ1AdvancedXL and EZ1 virus kit v2.0 (Qiagen, Hilden, Germany). Subsequently EBOV RNA was amplified either by a laboratory developed assay (LDA) or – as a back-up – using the RealStarFiloscreen kit v1 (Altona Diagnostics, Hamburg Germany). The in-house qRT-PCR assay was an internally controlled dual target assay, based on US-CDC NP and L gene primer sets, for which primer sets had been checked against sequences from the outbreak strain and validated in Erasmus MC according to ISO15189:2012 [8]. Briefly, 8 µl extracted nucleic acids were added to 12µl mastermix containing 0.4 µl primers and probes for each target (NP, L and PDV) and 5 µl 4x one-step fast virus mastermix (Lifetechnologies, USA) using white 96-wells plates. All master mixes were prepared in a clean room on the premises where the laboratory container was placed. The 96-wells plate was centrifuged briefly in a salad spinner (Ikea, Sweden). The qRT-PCR was run in a LightCycler96 using a cycling profile of 5 min. 50°C, 20 sec 95°C followed by 45 amplification cycles of 3 sec 95 °C and 30 sec 60°C each. Acquisition was done at the beginning of the 60°C annealing/elongation step, and fluorescence was read in channel 1, 470-514nm (FAM, L-gen), channel 2, 533-572nm (Dragon-fly orange, NP-gen) and channel 4, 645-697nm (Cy5, PDV). Total cycling time was 1:05 hours. For QA purposes the seals on the plates were meticulously checked for breaches directly after each run and the plates were immediately discarded in a separate sealed waste bag. In addition the working place for the extraction robot and the PCR machine were thoroughly cleaned with 1000 ppm hypochlorite solution on a regular basis and extra when deemed necessary. The RealStar Filovirus screen real-time RT-PCR was performed according to manufacturer’s instructions using 10µl extracted nucleic acids in a total volume of 30µl. Total run-time was 2.5 hours.
Quality assurance
A negative-, positive-, and internal process control (NPC, PPC, IPC), allowed the performance monitoring of each step of the complete process. Inactivated whole Ebola virus particles (kindly provided by the ENIVD network) was used as PPC, while an inactivated phocine distemper virus (PDV) was used as IPC [7]. Ct values were monitored for the IPC and PPCs to determine internationally accepted quality control cut-off values; Ct values should be within the 3 times the standard deviation from means (SD) range for a result to be accepted. Quality control charts were used to enter all data and to determine the correct cut-off value. Specimens were considered positive if the following conditions were met (1) the EBOV- L and/or NP target(s) were positive (Ct<40), (2) the IPC and PPC were positive and gave results within +/- 3SD of the mean and (3) the NPC was negative. Samples that were not interpretable because of inhibitory PCR conditions were retested using a 1:10 dilution of the extracted total nucleic acids. If PPC Ct value was >3SD the run was repeated. Results were scored indeterminate if upon repeated testing both the EBOV targets were negative and the IPC was out of range; a new sample was then requested. Results were interpreted with knowledge on the date of onset of clinical symptoms, date of specimen collection, and the case history and supported by consultation with the responsible clinician unless the samples were body swabs [3].
Before the start of diagnostic operations, all laboratories had to report the results of a blinded external quality assessment control panel, provided by the WHO/ European Network for Imported Viral Diseases to Erasmus MC. Laboratories became operational when achieving a 100% score. During operations data generated by the laboratories in the field was monitored for inconsistencies and unusual patterns by Erasmus MC.
Data collection & communication
All data were handled electronically. The sample forms were barcode labelled, date/time stamped, photographed and sent by a local network to a dedicated laboratory laptop. The details of the complete processing of each sample (Figure 2) was recorded into an excel file (Microsoft, USA) standardized for all laboratories in Sierra Leone and Liberia and reported on a daily basis according to national requirements. All steps were double checked by a second technician. Data were regularly stored on an external hard drive to prevent data loss in case of a computer crash. Results and clinical data were added into the excel file. In case of a positive result the District Ebola Response Centre (DERC) was directly informed to enable a rapid response.
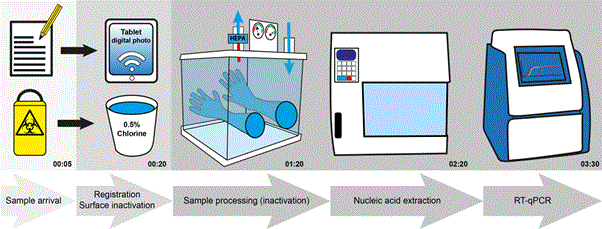
Figure 2. Schematic presentation of sample workflow and time-line of Dutch Mobile laboratories in West Africa, 2015 for ~ 10 samples. Step 1: Sample arrival: check biosafe containment and administration (5 min.). Step 2: Clinical forms photocopied for further administration and decontamination with 0.5% hypochlorite (15 min.). Step 3: Decontamination inner package in 1% hypochlorite, inactivation sample for EBOV testing, and Malaria testing (if whole blood) in Biosafety level 3 cabinet (75 min.). Decontamination sample tubes for EBOV testing for export out of Biosafety cabinet. Step 4: Automated nucleic acid extraction (60 min. including set-up of the machine). Step 5: Ebola virus real-time RT-PCR (70 min). Step 6: Interpretation PCR data including Quality Assurance check, administration and result reporting to responsible clinician (not shown, 30 min.).
Volunteers and training
To staff the laboratories, a call was sent out to all human and veterinary diagnostic laboratories in the Netherlands. Selection of volunteers was based on their previous experience with human clinical diagnostics, molecular techniques, gloveboxes and BSL3 laboratories. Teams consisted of 3-5 members comprising at least one virologist/microbiologist, one experienced technician in molecular diagnostics and one experienced BSL3 worker, supplemented with routine diagnostic laboratory technicians. Volunteers were interviewed/assessed by technicians, biosafety experts, diagnostic experts, scientists experienced in working with high threat pathogens, and managers/team leaders of ErasmusMC. Training was provided by Erasmus MC with support of the NGOs Save the Children and Medicines Sans Frontieres (MSF) included a) EBOV background and epidemiology, b) EBOV diagnostics: general background and potential issues, c) background and technicalities of Dutch labtainers, d) PPE donning/doffing, e) BSL3 glove-box work theory and practice, f) sample receipt/administration practice, g) nucleic acid extraction and PCR theory and practice, h) malaria diagnostics by antigen tests theory and practice, i) data interpretation and management, j) quality control and k) spill protocols, l) team roles and team discipline. Part of the training took place in a mock laboratory built for this cause at Erasmus MC with supportive materials like step-by-step photo instructions, dedicated Standard Operating Procedures (SOP) and manuals. In addition topics like l) essential country, culture and safety information, m) EVD in local context and n) personal resilience were addressed by the NGOs Save the Children or MSF in a two-day course in London and through a six-hour on-line course by the organization Disaster-Ready. The final teams were composed based on consensus in the group of interviewers and included performance of the team during training. At location a three day overlap was scheduled between subsequent teams for transition and on-site training. During the deployment, staff from Erasmus MC were 24/7 available for assistance and troubleshooting. Feedback through teleconferences was used to update protocols based on local experience of the teams during implementation. A data-sharing platform was used to support communication between trainers, volunteers and to provide access to all necessary (updated) documents. Visa, insurances and emergency repatriation were arranged by the Dutch government. In general each team worked 10-12 hours per day, 7 days a week for a period of 4-5 weeks.
Results
Laboratory placement
On the 6th of November 2014, three mobile laboratories were shipped by the Dutch marine transport vessel JSS Karel Doorman to West Africa, where they arrived two weeks later. The first laboratory (ownership DFID) was deployed to Koidu, a village in the Eastern province Kono, Sierra Leone (Figure 3a, lab #24). Since early December 2014, Kono province had been a hot spot area for active EBOV transmission. This region borders the Gueckedou province in Guinee where the EBOV epidemic started with a spill-over event of the virus from wildlife to humans. Prior to the deployment, no local laboratory capacity was available and samples had been flown by helicopter to Bo district for analysis, resulting in a turn-around-time (TAT) of > 3 days. Staff and local logistics were embedded with PIH while the lab was operational within the Wellbody Alliance/Red cross. After passing import inspection, the laboratory arrived at location on December 29, 2014 and was operational on January 13, 2015. It served a nearby Ebola Treatment Unit (ETU), several Community Care Centres (CCC) and regional burial teams.
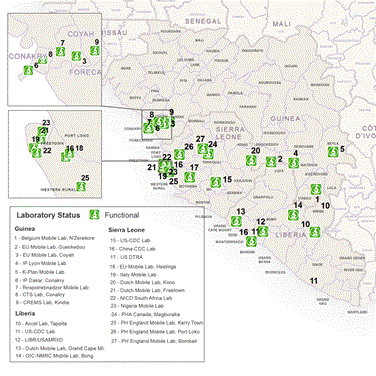
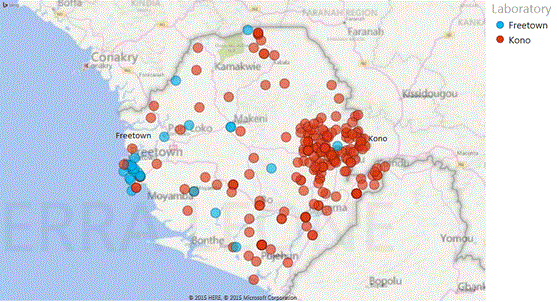
Figure 3. Location and catchment area Dutch Mobile Laboratories in West Africa, 2015. (A) Overview location and total spectrum of Ebola virus laboratories in Sierra Leone and Liberia as reported by WHO in February 2015. Dutch laboratories are represented by #13 (Sinje, Liberia) and # 20 (Kono, Sierra Leone) and #21 (Freetown, Sierra Leone). Source: WHO situation report 25 March 2015 (http://apps.who.int/ebola/current-situation/ebola-situation-report-25-march-2015). (B) Overview of catchment area two Dutch mobile laboratories in Sierra Leone based on village of residence of patients of which samples were tested. Residence data Sinje, Liberia were uninterpretable.
The second laboratory (ownership MOH Liberia) was deployed to Sinje, a village in the province Grand Cape Mount, Liberia (Figure 3a, lab # 9). The laboratory was placed near an ETU, operated by IOM. Prior to this deployment, no local laboratory capacity was available in the Grand Cape Mount area and TAT was >3 days. The lab was operational on January 30, 2015. The laboratory was asked to serve the Sinje ETU, an ETU in the bordering province Bombi and swabs taken by regional burial teams. Staff and local logistics were embedded with IOM.
The third laboratory (ownership MOH Sierra Leone) was deployed to the campus of the Princess Christian Maternity Hospital and the Ola during Children Hospital in Freetown, the capital of Sierra Leone in the province Western Urban (Figure 3a, lab # 25). Both hospitals were the only facility with this specialism in the country. Pregnant women and children under age of 5 years that presented themselves with putative signs of EVD were rejected for admission to the hospitals and were transferred to holding units on the premises pending their EBOV test results. Emergency Caesarean- sections and specialized care were not performed, and TAT before the presence of the Dutch Mobile laboratory was an average 3 days, too long to provide proper care and resulting in unnecessary deaths. After passing the necessary clearance, the laboratory was placed at the final location on February 11 and became operational on February 17, 2015. The two laboratories in Sierra Leone had a broad survey area based on residence addresses of patients (Figure 3b).
Laboratory output
The Kono-laboratory was operational from 13 January – 2 August 2015 and run by a total of 8 teams. The Sinje-laboratory was operated by 3 teams from 30 January- 17 March 2015 and the Freetown-laboratory with 7 teams from 18 February – 18 August 2015. In total, 2983 specimens were tested in Kono, 139 in Sinje and 3271 in Freetown. The number of specimens tested per day varied from 1 (Sinje) to 47 (Freetown). The average daily sample load was 15.3 in Kono, 3.2 in Sinje and 18,5 in Freetown (Table 1, Figure 4). The majority of the total of 6393 samples were labelled as initial samples (74.4%) representing 4756 individual patients The average age of the patients was 17.9 years, and varied significantly between the laboratories (Mann Whitney test,P <0.005), with 6.8 years for Freetown, 30.6 for Kono and 37.4 for Sinje. The age distribution for each laboratory is given in Table 1. Overall, comparable numbers of males vs females were tested in all three laboratories (Chi2 test, P<0.005) (Table 1).
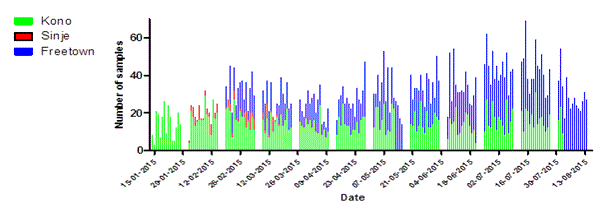
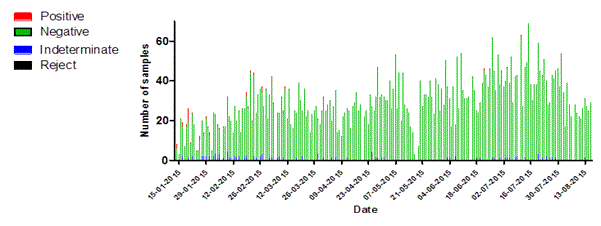
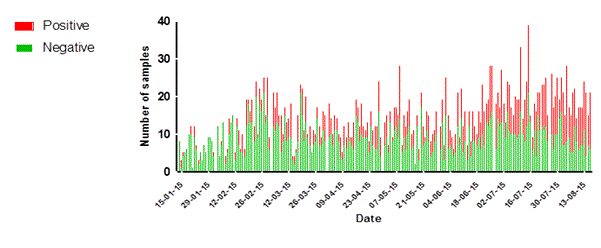
Figure 4. Statistics of daily sample numbers Dutch Mobile Laboratories, West Africa, 2015. (A) The combined number of specimens tested for each laboratory per day. (B) The number of specimens tested per day by EBOV test result for the laboratories combined. (C) The number of specimens tested per day by Malaria test result for the three laboratories combined.
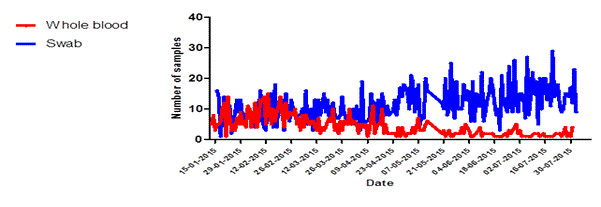
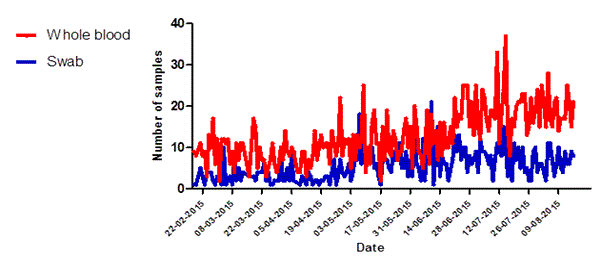
Figure 5. Specimen type received at Dutch Mobile laboratories in Sierra Leone, 2015. (A) Kono, and (B) Freetown.
Table 1. The age distribution for each laboratory
|
Column1
|
Column2
|
Column3
|
Column4
|
Column5
|
Column6
|
Column7
|
Column8
|
Column9
|
Column10
|
|
Cohort
|
|
Konoⱡ
|
Sinjeⱡ
|
Freetownⱡ
|
Totalⱡ
|
|
Gender
|
Female
|
1213
|
40.7%
|
52
|
37.4%
|
1694
|
51.8%
|
2959
|
46.3%
|
| |
Male
|
1569
|
52.6%
|
71
|
51.1%
|
1383
|
42.3%
|
3023
|
47.3%
|
| |
Unknown
|
201
|
6.7%
|
16
|
11.5%
|
194
|
5.9%
|
411
|
6.4%
|
| |
Total
|
2983
|
|
139
|
|
3271
|
|
6393
|
|
|
Age (years)*
|
average
|
30.6 (0-135)
|
|
37.4 (0-105)
|
|
6.8(0-105)
|
|
17.9 (0-135)
|
|
|
Age groups (years)
|
0
|
651
|
21.8%
|
11
|
7.9%
|
1047
|
32.0%
|
1710
|
26.7%
|
| |
1-14
|
377
|
12.6%
|
14
|
10.1%
|
1537
|
47.0%
|
1627
|
25.4%
|
| |
15-44
|
1096
|
36.7%
|
71
|
51.1%
|
523
|
16.0%
|
1990
|
31.1%
|
| |
45+
|
782
|
26.2%
|
39
|
28.1%
|
43
|
1.3%
|
864
|
13.5%
|
| |
Unknown
|
77
|
2.6%
|
4
|
2.9%
|
121
|
3.7%
|
202
|
3.2%
|
|
Samples
|
|
|
|
|
|
|
|
|
|
|
total # and type
|
Live swab
|
4
|
0.1%
|
0
|
0.0%
|
23
|
0.7%
|
27
|
0.4%
|
| |
Swab
|
2184
|
73.2%
|
81
|
58.3%
|
943
|
28.8%
|
3208
|
50.2%
|
| |
Whole blood
|
795
|
26.7%
|
58
|
41.7%
|
2305
|
70.5%
|
3158
|
49.4%
|
| |
Total
|
2983
|
|
139
|
|
3271
|
|
6393
|
|
|
# per day*
|
Average
|
15.3 (30-31)
|
|
3.2 (1-9)
|
|
18.5 (3-47)
|
|
29.6 (3-69)
|
|
|
Sample status
|
Initial
|
1809
|
60.6%
|
115
|
82.7%
|
2832
|
86.6%
|
4756
|
74.4%
|
| |
Follow-up
|
352
|
11.8%
|
0
|
0.0%
|
34
|
1.0%
|
386
|
6.0%
|
| |
Repeat
|
12
|
0.4%
|
24
|
17.3%
|
166
|
5.1%
|
202
|
3.2%
|
| |
Unknown
|
810
|
27.2%
|
0
|
0.0%
|
239
|
7.3%
|
1049
|
16.4%
|
|
EBOV results**
|
Positive samples
|
34
|
1.1%
|
2
|
1.44
|
17
|
0.52
|
53
|
0.8%
|
| |
Negative
|
2872
|
96.3%
|
132
|
94.96
|
3240
|
99.05
|
6244
|
97.7%
|
| |
Indeterminate
|
69
|
2.3%
|
5
|
3.6
|
7
|
0.21
|
81
|
1.3%
|
| |
Rejected
|
8
|
0.3%
|
0
|
0
|
7
|
0.21
|
15
|
0.2%
|
|
Unique EBOV positives per age group
|
0
|
1
|
|
|
|
2
|
|
3
|
6.5%
|
| |
1-14
|
6
|
|
|
|
8
|
|
14
|
30.4%
|
| |
15-44
|
18
|
|
1
|
|
6
|
|
25
|
54.3%
|
| |
45+
|
2
|
|
1
|
|
|
|
3
|
6.5%
|
| |
Unknown
|
1
|
|
|
|
|
|
1
|
2.3%
|
|
Malaria results
|
Negative
|
578
|
72.7%
|
39
|
67.2%
|
1343
|
58.4%
|
1960
|
62.1%
|
| |
Positive
|
208
|
26.1%
|
17
|
29.3%
|
954
|
41.4%
|
1179
|
37.4%
|
| |
Invalid
|
9
|
1.13%
|
2
|
3.4%
|
4
|
0.2%
|
15
|
0.5%
|
|
TAT received – result***
|
whole blood same day result
|
721
|
90.7%
|
38
|
65.5%
|
923
|
40.0%
|
1682
|
53.3%
|
| |
whole blood same or next day result
|
777
|
97.7%
|
57
|
98.3%
|
2249
|
97.6%
|
3083
|
97.6%
|
| |
swab same day result
|
2152
|
98.5%
|
NA
|
NA
|
625
|
66.3%
|
2777
|
86.5%
|
| |
swab same or next day
|
2184
|
100.0%
|
NA
|
NA
|
936
|
99.3%
|
3120
|
97.3%
|
|
TAT collection to result****
|
Whole blood same day result
|
721
|
90.7%
|
38
|
65.5%
|
923
|
40.0%
|
1682
|
53.3%
|
| |
whole blood same or next day
|
777
|
97.7%
|
57
|
98.3%
|
2249
|
97.6%
|
3083
|
97.6%
|
| |
swab same day result
|
253
|
12.2%
|
39
|
48.1%
|
542
|
57.5%
|
834
|
27.1%
|
| |
swab same or next day result
|
1794
|
86.5%
|
72
|
88.9% 2021 Copyright OAT. All rights reserv
|
923
|
97.9%
|
2744
|
89.0%
|
| |
|
|
|
|
|
|
|
|
|
|
Ebolavirus disease diagnostics
Of the 6393 samples tested at the three laboratories, 53 (0.82%) were positive for EBOV. In Kono 34 specimens of 28 individual patients were positive, in Sinje 2 specimens of 2 patients and in Freetown 17 samples of 16 patients. EBOV RNA was detected in 42 blood and 11 swab specimens. The majority (54%) of the EBOV positive samples was found in the age group of 15-44 years while this group comprised 31% of the total number of samples submitted for analysis. Although 26.7% of the samples submitted for EBOV analysis were from new-borns (< 1 years of age), only 6.5% of the positive samples were found in this age category (Table 1).
Of the 81 samples that could not be interpreted 15 were whole blood samples (0.47% of n= 3158) while 66 were swabs from deceased persons (2.1% of n=3208) (data not shown). In the first three months of operations the Kono-laboratory received an equal proportion of whole blood samples and swabs. However after the identification of the last EVD patient in the region on 10 March, 2015 swabs became gradually the main type of sample offered for testing (figure 5a). In Freetown the number of daily whole blood samples kept increasing over time while the number of swabs remained stable (Figure 5b).
Malaria diagnostics
In total, 3154 whole blood specimens were tested for P. falciparum malaria at the three laboratories, of which 99.5% (3139) gave a valid result (Table 1). The percentage of positive specimens varied per laboratory, with 26.1% in Kono, 35.4% in Sinje and 41.4% in Freetown. Malaria diagnostic results over time are given in figure 4c. 71% of the positive malaria patients were in the age group of 1-14 years old which represented 25% of the total number of samples. In the age group > 45 years of age (14% of the total patient population) < 1% of the positive malaria cases were found while in the category new-borns 10% of the submitted samples were malaria positive (data not shown).
Turn-around-time
For the majority of whole blood and swab specimens the results were reported on the day of sample receipt by the laboratory in Kono and Freetown; respectively 65.3% and 66.3% in Freetown and 95.1% and 98.5% in Kono. > 99% of the samples were reported within 24 hours upon receipt (Table 1). These data were not registered for Sinje. The reported times between collection of the sample from the patient to the EBOV test results, which takes into account the transportation time of the samples to the laboratories, show that > 97% of the whole blood samples were reported within 24 hours upon sample collection in Kono (97.7%), Sinje (98.3%) and Freetown (97.6%) although the generation of results on the day of sample collection was far less frequent in Sinje (65.5%) and Freetown (40%) owing to the timing of sampling teams. The same patterns were observed for swabs from dead bodies in Sinje and Freetown, whereas there was a clear delay between the collection of swabs and the generation of test results in Kono, where only 12.2% could be tested on the day of collection as swabs were often collected from bodies at remote locations. (Table 1)(Chi2test, P< 0.05).
Multiple EBOV diagnostic laboratories were operational in Freetown. To have a better view and grip on the timeliness of the laboratory activities, the Laboratory Technical Working Group who monitored laboratory activities in Sierra Leone, requested that all laboratories operational in Freetown would record the exact time of sample reception and reporting. The median recorded times were 5.58 hours for whole blood and 5.83 for swabs for the Dutch Freetown laboratory (data not shown).
Discussion
Three Dutch laboratories have successfully supported the national and international efforts to control the EVD epidemic in West-Africa; each in a specific setting with respect to the size and type of catchment area.
The majority of the EBOV positive samples was found in the age group of 15-44 years which is in line with WHO observations till August 12, 2015 [9]. The average age of the EBOV patients was significantly lower for the Freetown laboratory that supported the national children’s hospital. Rapid TATs are critical for individual patient care, timely contact tracing and an adequate surveillance program. The average TAT (sample receipt to result) in the catchment areas of the three laboratories was reduced from 3-4 days to < 24 hours (> 97.6% of the samples) by the operations of the Dutch laboratories. The TAT from actual sampling to results for dead body swabs in Koidu was relatively long when looking at the fraction of samples that yielded results on the same day (12.2%). This reflects the submission to the Koidu laboratory of a substantial amount of samples of dead bodies from remote areas and from community care centers at greater distances from the Koidu laboratory than the holding centers and ETUs that were mainly served by the laboratories in respectively Freetown and Sinje.
In the 8th week of operations the Koidu laboratory identified the last EVD patient in the Kono region. This marked the region’s beginning of the transition phase in which primary diagnostics for patient triage was gradually replaced by surveillance testing. This is reflected in the type of samples received in Koidu in time. In the transition/surveillance phase swabs from deceased persons became gradually the main type of sample offered for testing. In contrast in the Freetown West-urban area positive cases continued to be identified, with the last positive case identified in the Dutch laboratory on the 7th of August with the laboratory closing its operations only 10 days later. The continued circulation of EBOV in the Freetown area was reflected in the sample type submitted for testing. The number of daily whole blood samples kept increasing over time while the number of swabs remained stable.
During the 6 month period in which the Dutch Mobile Laboratories operated, a substantial increase in positive test result for malaria antigen tests was observed (Fig. 4c). In the area in which the laboratories operated, malaria is highly seasonal with cases mainly presenting between July and December [10], which might explain the observed increase over time.
Besides rapid EBOV and malaria testing the Dutch laboratories in Sierra Leone supported tracing of remaining EBOV transmission pockets by preparing samples for the Wellcome Trust sequence facility in Kenema for real-time monitoring of circulating EBOV diversity. Forty-eight EBOV whole genome sequences from 44 patients were determined. The sequencing results of the last 4 patients detected in the Freetown laboratory, results were obtained and reported to WHO within two weeks of sample collections confirming a chain of transmission in Freetown’s Magazine Warfs area [11].
The container-based construction proofed deployable even to more remote areas like Sinje and Koidu and sustainable in the dry and the wet season while the laboratory set-up delivered state-of-the art EBOV and malaria diagnostic testing capability. The technical platforms were chosen as such to facilitate easy implementation of other molecular diagnostic tests, including for measles and Lassa fever which were already validated to this goal on the platforms at Erasmus MC. The successful training of local personnel by the Dutch teams, who are currently running the laboratories in Sierra Leone demonstrate the potential of the laboratories to link within a sustainable laboratory infrastructure that currently needs to be established in West-Africa in the final phase of the EBOV outbreak.
In summary, we describe the set-up and operations of the three Dutch mobile laboratories during control of the EVD outbreak in West-Africa. Multiple laboratories with different set-up were deployed during the outbreak [8,12-14]. Future emergency deployments would benefit from a “lessons learned” exercise through an assessment and comparison of all logistic, technical and human aspects of the deployment of these laboratories.
Acknowledgements
We thank the hospital staff of PCMH, ODCH, and Koidu and Sinje ETUs for their efforts in the Ebola virus outbreak. We thank the Office of the President Sierra Leone, the Sierra Leone and Liberian MOH, PIH and OIM for continuous support and efforts in the Ebola virus outbreak response. We thank the drivers, pilots, non-governmental organizations, district medical and surveillance officers for their help with sample collection and logistics in Sierra Leone. We thank the volunteers and their employers: Adam Hume, Amber Hendriks, Anne van der Linden, Annelies Mesman, Annemieke Dinkla, Arie Kant, Bart Kooi, Bas van der Veer, Chantal Reusken, Cindy Warringa, Eline Verheij, Ellis Meuleman, Emily Nelson, Emre Kucukkose, Erik van Hannen, Evelien Kern, Felix Geeraedts, Florine Scholte, Gudrun Freidl, Guido van der Net, Hans Naus, Harry Vennema, Heidi De Gruyter, Hein Sprong, Heleen Klos, Henny Pieterse Bruins, Irma Verhoofstad, Jacqueline Schelfaut, Janienne Klaasse, Janke Schinkel, Janko van Beek, Jelke Fros, Jennifer de Jong, Jeroen Cremer, Jeroen Roose, Jet Kant, Joffrey van Prehn, Jolanda Maaskant, Judy Yen, Jurre Siegers, Katja Wolthers, Lesla Bruijnesteijn, Ludo Oostendorp, Marion Nederpel, Marion Sunter, Marloes Dullaart, Martine Slop, Matthew McCall, Nancy Beerens, Naomi Berkeveld, Nathalie van Burgel, Nicole van Manen, Nienke van Teijlingen, Nina Hertoghs, Petra van den Doel, Pieter Smit, Richard de Boer, Robbert Bentvelsen, Robin van Houdt, Ruud Jansen, Saskia Bierman, Seta Jahfari, Stefan Boers, Stephanie Blanc, Suzan Pas and Viviana Cobos. Logistics and training team at ErasmusMC: Jolanda Maaskant, Judith Guldemeester, Janine Klaas, Chantal Burghoorn-Maas, Anouk Gideonse, Hans Kruining, Saskia Smits, Rogier Bodewes, Carolien van de Sandt, Robin Huisman, Suzan Pas, Marion Koopmans, Bart Haagmans, Chantal Reusken, Heidi de Gruyter, Guido van der Net, Jaap van Hellemond. Furthermore the team at Hospitainer. Finally Derk de Haan (MOFA) for all logistic assistance and SAVE the Children and Doctors Without Borders for training support. Finally, the board of directors of ErasmusMC for support of the deployment after termination of financial support by the Dutch government on June 15, 2015.
References
- WHO (2015) WHO: Ebola Response Roadmap Situation Report 9 December 2015.
- WHO (2015) WHO: Ebola Response Roadmap Situation Report. 25 March 2015.
- Reusken C, Niedrig M, Pas S, Anda P, Baize S, et al. (2015) Identification of essential outstanding questions for an adequate European laboratory response to Ebolavirus Zaire West Africa 2014. J Clin Virol 62: 124-134. [crossref]
- Hospitainer.com. Hospitainer, rapid deployment hospital: Hospitainer, 2016.
- Goodfellow I, Reusken C, Koopmans M (2015) Laboratory support during and after the Ebola virus endgame: towards a sustained laboratory infrastructure. Euro Surveill 20: 12.
- CDC. Guidance on Personal Protective Equipment (PPE) To Be Used By Healthcare Workers during Management of Patients with Confirmed Ebola or Persons under Investigation (PUIs) for Ebola who are Clinically Unstable or Have Bleeding, Vomiting, or Diarrhea in U.S. Hospitals, Including Procedures for Donning and Doffing PPE 2015.
- Hoek RA, Paats MS, Pas SD, Bakker M, Hoogsteden HC, et al. (2013) Incidence of viral respiratory pathogens causing exacerbations in adult cystic fibrosis patients. Scand J Infect Dis 45: 65-69.
- Flint M, Goodman CH, Bearden S, Blau DM, Amman BR, et al. (2015) Ebola Virus Diagnostics: The US Centers for Disease Control and Prevention Laboratory in Sierra Leone, August 2014 to March 2015. J Infect Dis 2: S350-S358.
- WHO (2015) WHO: Ebola Response Roadmap Situation Report 12 August 2015 2015.
- Walker PG, White MT, Griffin JT, Reynolds A, Ferguson NM, et al. (2015) Malaria morbidity and mortality in Ebola-affected countries caused by decreased health-care capacity, and the potential effect of mitigation strategies: a modelling analysis. Lancet Infect Dis 15: 825-832.
- Smits SL, Pas SD, Reusken CB, Haagmans BL, Pertile P, et al. (2015) Genotypic anomaly in Ebola virus strains circulating in Magazine Wharf area, Freetown, Sierra Leone, 2015. Euro Surveill 20. [crossref]
- Lu HJ, Qian J, Kargbo D, Zhang XG, Yang F, et al. (2015) Ebola Virus Outbreak Investigation, Sierra Leone, September 28-November 11, 2014. Emerg Infect Dis 21: 1921-1927. [crossref]
- Chen Z, Chang G, Zhang W, Chen Y, Wang X, et al. (2015) Mobile laboratory in Sierra Leone during outbreak of Ebola: practices and implications. Sci China Life Sci 58: 918-921. [crossref]
- Wölfel R, Stoecker K, Fleischmann E, Gramsamer B, Wagner M, et al. (2015) Mobile diagnostics in outbreak response, not only for Ebola: a blueprint for a modular and robust field laboratory. Euro Surveill 20. [crossref]










