Background :Cardiogenic embolism accounts for 15 to 40 % of all ischemic stroke. In general, cardiogenic embolic stroke is a severe case and usually recurrent, treatment and prognosis are different from other strokes. Cardiac embolic strokes need to be diagnosed by imaging tools with evidence of cardiac resources of embolism. Transthoracic Echocardiography and Transesophageal Echocardiography are useful tools for detection of the cardioembolic resources.
Cardioembolic strokes may have symptoms and signs (atrial fibrillation, rheumatic heart disease, infective endocarditi) but may not have any symptom and sign even have normal result on transthoracic ehocardiography in some cardioembolic strokes (Patent Foramen Ovale, IAS aneurysm)
Purpose :By echocardiography (transthoracic and transesophageal) detection of cardiacembolic resources in suspected cardioembolic stroke patients.
Methods :Suspected cardiogenic embolic strokes were refered to our department to perfome echocardiography. All patients were determined cerebral infarction or TIA by neurologist with appropriate results of brain MRI or MSCT. Transthoracic Echocardiography was formerly performed, and Transesophageal Echocardiography followed latter in patients who had negative or unexplained findings on Transthoracic Echocardiography.
Results :63patients (59 strokes and 4 transient ischemic attacks) including 28 men and 35 women, mean age 49 ± 15. Transthoracic Echocardiography was done for all patients, Only 14 cases of Transesophageal Echocardiography were performed (2 cases of left atrial dilation due to intermittent atrial fibrillation, 12 cases of normal result on Transthoracic Echocardiography). In 2 cases with left atrial dilation we found spontaneous contrast in the LA and LAA. In 12 cases of normal result on Transthoracic Echocardiography, there were 9 cases of PFO, 1 case of PFO associated with IAS aneurysm and 2 normal results.
Conclusion :Beside major risk cardiogenic sources, echocardiography can detect underlying cardiac lesions of cardioembolic stroke. TTE has been shown to be effective in detecting potential cardiac sources at ventricles. TTE associated with TEE obtain more effectiveness in detection of cardiogenic sources.
Stroke is the second leading cause of death worldwide behind ischemic heart disease, with 5.5 million stroke deaths in 2016 [1]. About 85% case of strokes are due to acute ischemia [2].
In the United States approximately 795 000 peoples experience strokes each year. On average, every 40 seconds a stroke is diagnosed, and every 4 minutes a stroke death occurs [3]. Besides from 24% to 49% stroke survivals have some level of disability [4], the cost of their care is substantial.
In south Vietnam, from a stroke data shows the incidence of 161/100.000 and the prevalence of 415/100.000 [5].
Cardioembolic stroke accounts for 15 to 40 % in ischemic stroke [6]. The incidences are different from reports from countries, for example 20% in United State,19% in Australia, 31% in France, 38% in Greece and 17% in Germany [7].
Usually recurrent, treatment and prognosis of cardiacembolic stroke are different from other strokes, it is important to determine whether strokes are due to cardiac source of embolism or not. There are many tools can be used for detecting cardioembolic resources but Echocardiography is the first choice technique that can show comprehensive informations of cardioembolic risk in stroke patients.
Detection of cardiacembolic resources in patients with suspected cardioembolic stroke by echocardiographic techniques (TTE and TEE).
63 patients including 59 strokes and 4 transient ischemic attacks ( TIA ) suspected cardiogenic embolism were refered to our department to perfome Echocardiography.
Instrument: Philips X MATRIX iE33 machine with TTE and TEE probes.
All patients were determined cerebral infarction or TIA by neurologist and MRI or MDCT imagings.
TTE was performed first, TEE was indicated latter in cases of doubtful or negative result on TTE.
Among 63 studied patients (59 strokes and 4 transient ischemic attacks) patients including 28 men and 35 women, mean age 49 ± 15. TTE was done all of these patients,TEE was indicated in 14 cases ( 2 cases of LA dilation due to intermittent atrial fibrillation, 12 cases with normal result on TTE ). In 2 cases with LA dilation we found spontaneous contrast in the LA and LAA. In 12 cases of normal results on Transthoracic Echocardiography, there were 9 cases of Patent Foramen Ovale, 1 case of PFO associated with IAS aneurysm and 2 cases with normal results (Table 1).
Table 1. Causes of cardioembolic strokes
Causes of cardioembolic strokes |
Patients (%) |
Rheumatic mitral stenosis |
23 (37%), in which: 18 cases (78%) with AF and 5 cases (22%) without AF |
Atrial fibrillation without mitral valvulopathy |
16 (25%) |
Patent Foramen Ovale (PFO) |
10 (16%)
(9 cases PFO and 1 case PFO with atrial septal aneurysm) |
Endocarditis |
3 (5%) |
Mitral Valve Prolapse |
3 (5%) |
LV thrombus |
2 (3%) |
LA myxoma |
1 (1.5%) |
Sick sinus sydrome |
3 (5%) |
Normal finding on ultrasound |
2 (3%) |
Our study shows the combination of TTE and TEE is useful for finding cardioembolic resources. We avoid performing unnecessary TEE (such as clear apical LV thrombus). The anterior and near structures of the heart may be appropriate for TTE in detecting thromboembolic sources but TEE is the advantage technique of choice in cases of deep thromboembolic origin in the posterior cardiac structures and in the thoracic Aorta (Table 2).
Table 2. TEE in comparison with TTE in detecting cardioembolic sources
Thromboembolic sources |
Detected by TTE |
Detected by TEE |
Spontaneous contrast |
12 |
18 |
LA thrombus |
12 |
15 |
LAA thrombus |
0 |
20 |
PFO |
3 |
10 |
Vegetation |
1 |
3 |
Myxoma |
1 |
1 |
LV thrombus |
2 |
- |
Mitral stenosis |
22 |
22 |
Mitral valve prolapse |
3 |
3 |
Cardioembolic sources also may be detected by TTE in some cases, TEE is performed in patients with unclear results on TTE or get more informations for guiding therapeutic decision.
Because recurrence of strokes, treatment and prognosis of cardiaoembolic strokes are different from other strokes, early diagnosis of stroke from cardiac embolic sources is very important for treatment as well as secondary prevention.
In symptomatic patients, ECG and TTE can diagnose cardioembolic stroke. TEE provide usefull informations for treatment like detecting thrombi in LAA prior to cardioversion of AF or percutaneous balloon mitral valvuloplasty in mitral stenosis. In case of asymptomatic patients, TEE is helpful for diagnosis as well as treatment
High risk of cardioembolic strokes
Atrial Fibrillation: Atrial fibrillation (AF) is the most common cardiac arrhythmia, with prevalence of 1 % in the general population and increasing with age [8]. The incidence of stroke in atrial fibrillation patients is about from 2 to 10.5% per year depending on risk factors [9,10] including LA enlargement [11].
This arrhythmia is the most important resource responsible for 45% of cardioembolic stroke in Western countries [12] but in our data the rate is 25%.
TTE evaluates size and function of cardiac chambers, associated cardiac lesions such as valvular heart diseases, pericardial abnormalities. TEE evaluates size and morphology of LAA, velocity of LAA blood flow, identifies and excludes spontaneous contrast and thrombus of LAA and LA.
TTE can detect LA thrombus but the sensitivity is low. TEE is the credible technique to detect LA and LAA thrombi, with values of sensitivity and specificity about 99% [13].
In stroke patients due to atrial fibrillation may be no evidence of thrombus in LA or LAA but there is a decrease contractility and function of LAA, manifests as a low velocity of LAA blood flow and dilation of the LAA. In 4 types morphology of LAA (cactus, chicken wing, windsock and cauliflower), patients with non-chicken wing morphology of LAA are significant more likely to have embolic events after adjustment with other risk factors [14].
Rheumatic valvular diseases: Mitral stenosis is majority, stroke often occurs in mitral stenosis patients with atrial fibrillation, LA enlargement, spontaneous contrast, absence or presence of thrombus.
Rheumatic heart disease has the highest rate of cardioembolic stroke in our data 37% but only 12.4% in develop countries [15].
Vietnam as well as other developing countries lack of good socioeconomic and environmental conditions in previous decades leading to persistence this disease [16], while almost eradicated in developed countries [17].
TTE identifies the disease. TEE detects spontaneous contrast and thrombi in LA, LAA of patients with mitral stenosis. It is important to decide using anticoagulation before percutaneous balloon mitral valvuloplasty or surgery (Figures 1-8).
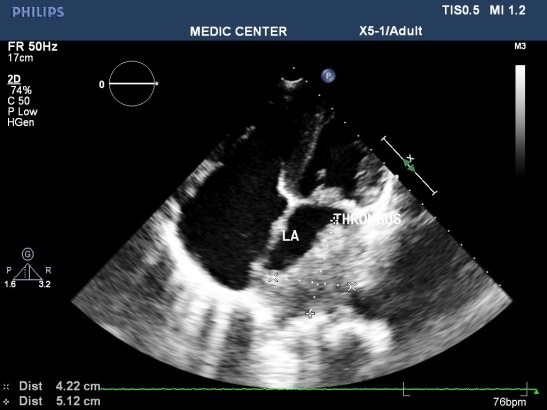
Figure 1. Mitral stenosis with thrombus of LA; “TTE image from Nguyen Tuan Vu”
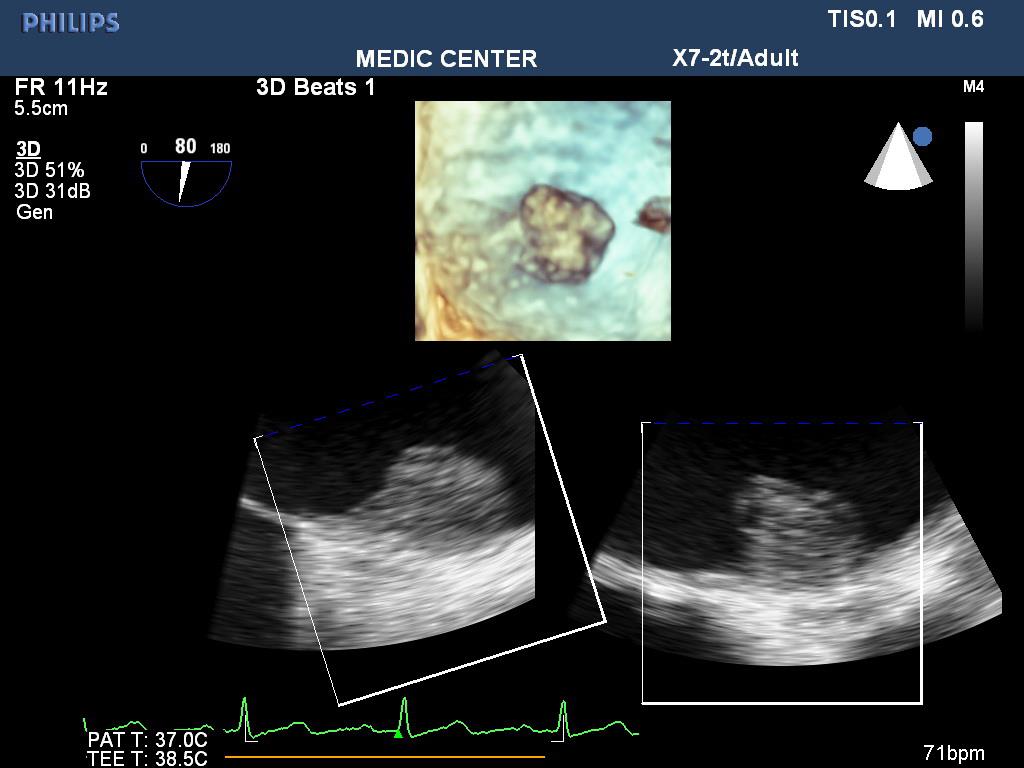
Figure 2. LA thrombus on 3 D TEE; “TEE image from Nguyen Tuan Vu”
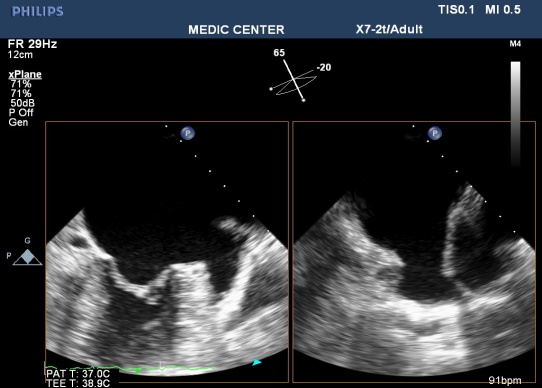
Figure 3. Normal LAA on TEE; “TEE image from Nguyen Tuan Vu”
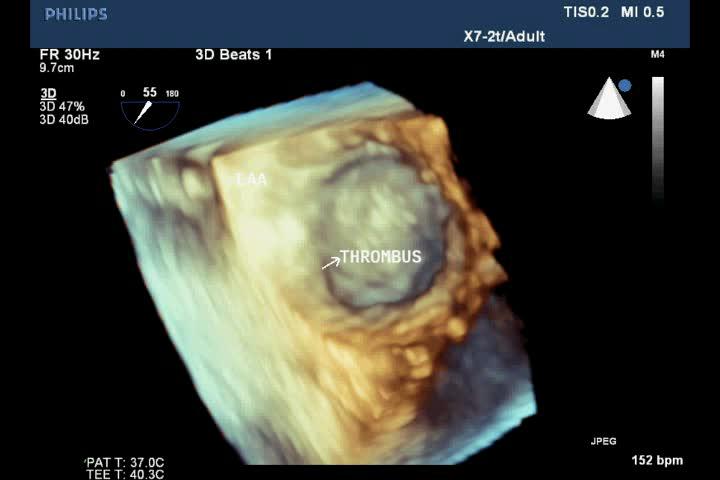
Figure 4. Thrombus in LAA on 3D TEE; “TEE image from Nguyen Tuan Vu”
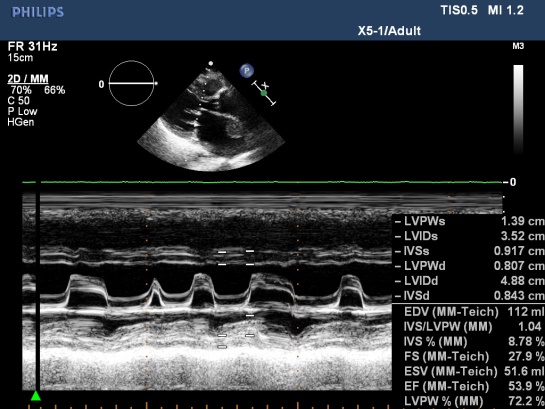
Figure 5. MS with AF, M-mode TTE; “M –mode TTE image from Nguyen Tuan Vu”
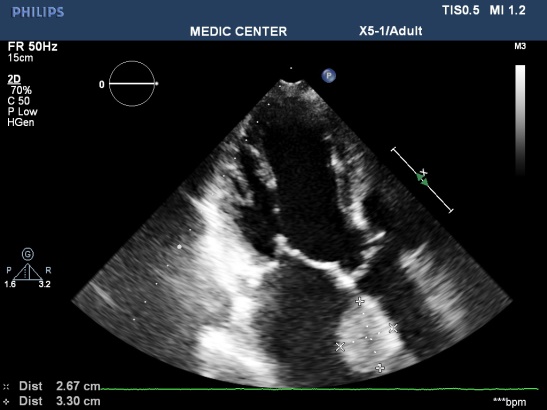
Figure 6. LA Thrombus on apical 3C view; “TTE image from Nguyen Tuan Vu”
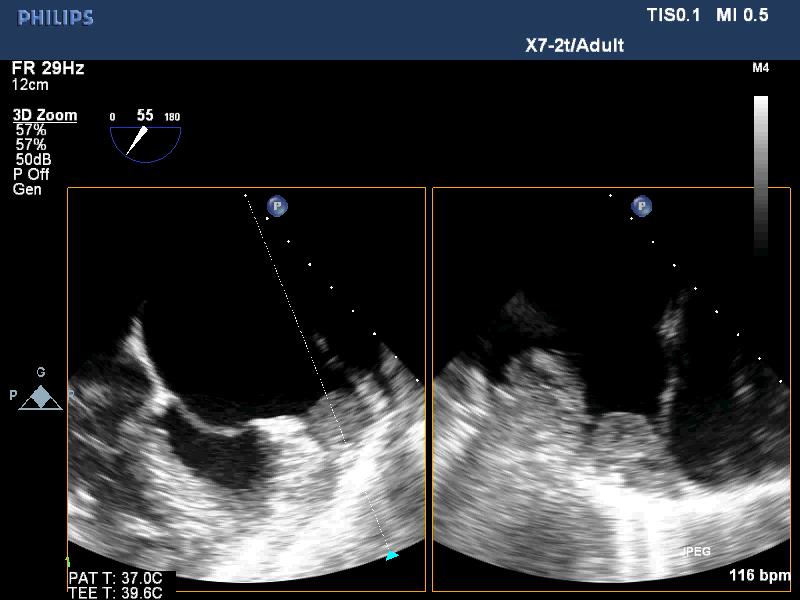
Figure 7. LAA thrombus, TEE images; “TEE image from Nguyen Tuan Vu”
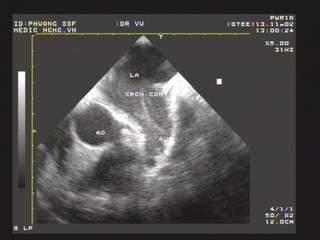
Figure 8. Spontaneus contrast in LAA; “TEE image from Nguyen Tuan Vu”
Left ventricular thrombus: In the context of old myocardial infarction, LV aneurysm, ischemic cardiomyopathy and dilated cardiomyopathy. In our data of 2 cases of LV thrombus including 1 case of old MI and 1 case of ischemic cardiomyopathy.
Features of LV thrombus on echocardiography: a mass with dense echogen, defined margin located adjacent endocardium but distinct from endocardium, existing at least in 2 views (Figure 9).
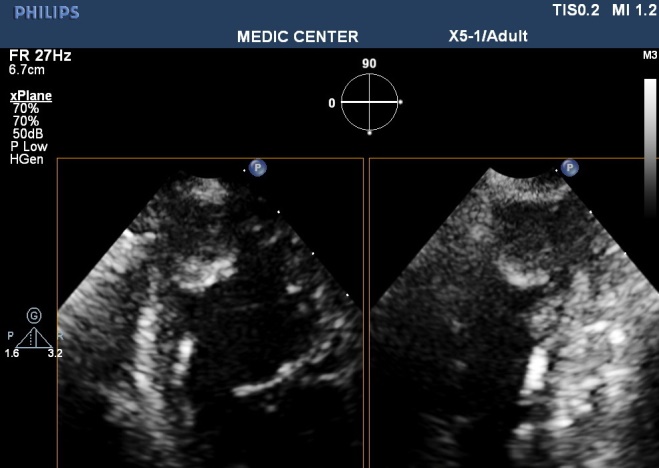
Figure 9. Apical thrombus in patient with MI; “TTE image from Nguyen Tuan Vu”
Cardiac mass: The two common cardiac tumors causing cardiac embolic stroke are myxoma and papillary fibroelastoma. Myxoma is the most common, located 75% in LA, 20% in RA and 5% in 2 ventricles with equal distribution [18].
Myxoma is responsible for 1% of stroke in the young. Cardiogenic embolism occurs 40-50% patients, caused by detached thrombus from the myxoma, rarely by tumor fragments.
In echocardiography, feature of myxoma is a mobile mass attached to the endocardial surface by a pedicle, usually arising from the fossa ovalis.
Majority myxoma may be detected by TTE, in cases of tumors with small size or those that involve finding site of pedicle attachment, TEE should be required for diagnosis.
We have a case of myxoma, no case of papillary fibroelastoma (Figures 10-13).
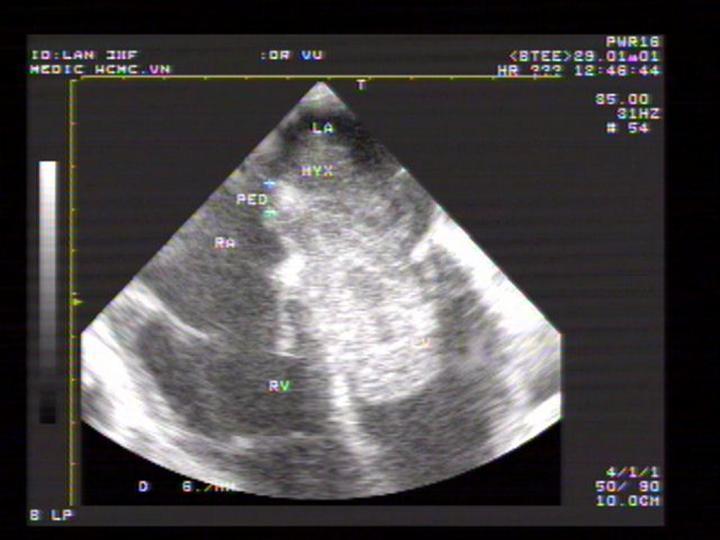
Figure 10. A giant LA myxoma attached to IAS; “TEE image performed by Nguyen Tuan Vu”
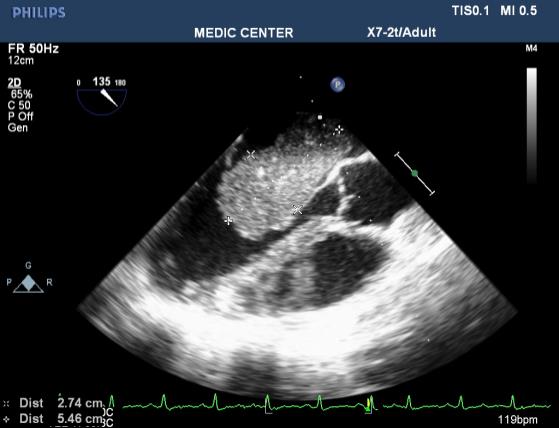
Figure 11. LA myxoma recorded on 135₀ view TEE; “TEE image performed by Nguyen Tuan Vu”

Figure 12. LA myxoma demonstrated by 3D TEE; “Image recorded by Nguyen Tuan Vu”
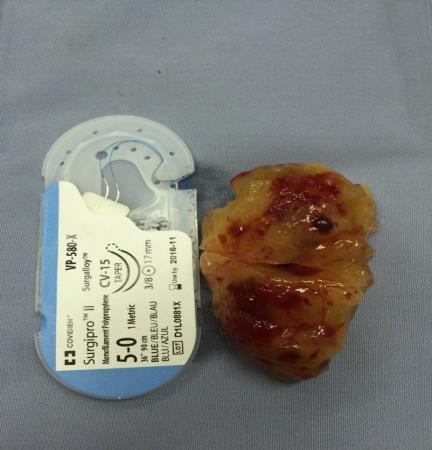
Figure 13. The characteristic findings of this myxoma
Infective Endocarditis: Embolic events occur in 22% to 50% of cases of infective endocarditis [19]. The incidence of cardioembolic strokes due to endocarditis is from 15 to 20%, with majority of embolic events present in the first 7-10 days after admission [20]. Echocardiography plays an important role in prediction of embolic events [21].
Vegetation that located on mitral valve and more than 10mm in size is associated with high risk of stroke.
TTE is performed first, easily detecting vegetations more than 10mm, however TEE is mandatory in cases of doubtful TTE.
In our data, there are 3 cases with vegetation on mitral valves, in which 1 case with vegetation greater than 10mm in size (Table 3) (Figures 14 and 15) [22].
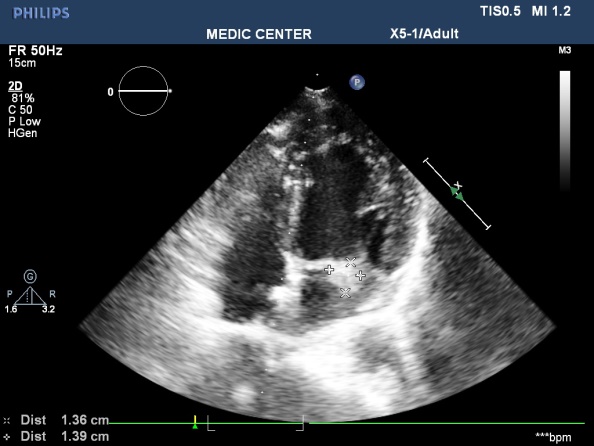
Figure 14. A gross vegetation attached to the PML; “TTE image done by Nguyen Tuan Vu”
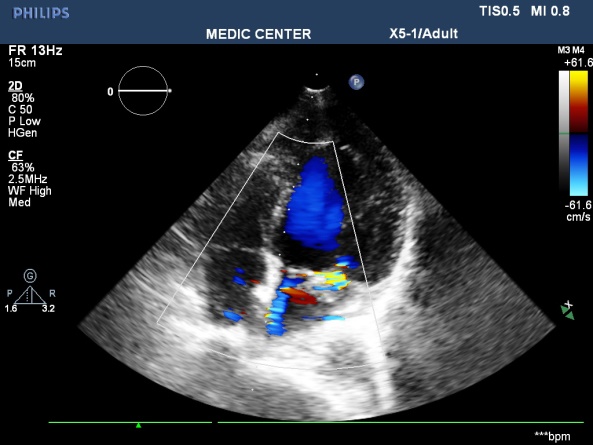
Figure 15. A excentric jet of MR due to PML prolapse; “TTE image done by Nguyen Tuan Vu”
Table 3. The specificity and the sensitivity of TTE and TEE [22]
Endocarditis |
TTE |
TEE |
Specificity |
98% |
100% |
Sensitivity |
44-60% |
88-100% |
Low risk of cardioembolic strokes
Atrial Septum Aneurysm: Atrial septum with redundant tissue bulging over 10mm into the right or left atrium or both atria in a heart circle creates atrial septal aneurysm. It is found in about 1 % [23] of consecutive autopsies and 4.9% of patients performed TEE for other reasons than indication for sources of emboli [24]. ASA is often associated with PFO, according to Schneider the rate is 77% [25], according to Silver & Dorsey the rate is 50% [26]. Data from Andreas M showed 54.4% shunt through atrial septum in patients with ASA [27]. Mechanisms of cardiogenic embolism consist of direct embolism from thrombus formation in aneurysm, paradoxal embolism through PFO or ASD from venous sources and stagnation of blood in aneurysm with poor contraction in atrial fibrillation [28]. We have a case stroke with atrial septal aneurysm associated with PFO detected by TEE (Figures 16-19).
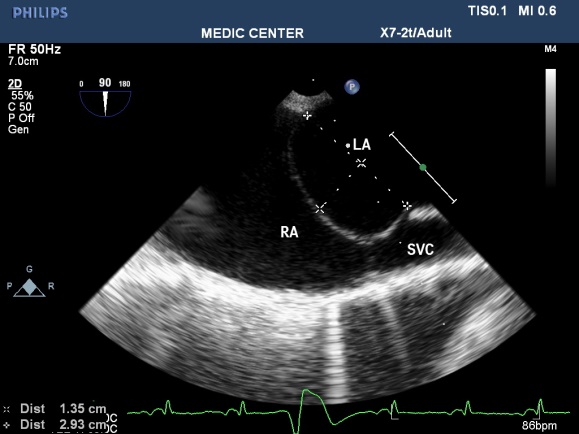
Figure 16. Sizing ASA by 2D TEE; “TEE image from Nguyen Tuan Vu”
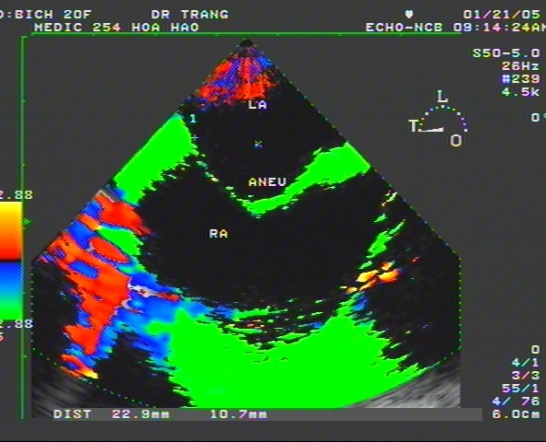
Figure 17. ASA bulging to the RA on TDI; “TDI image from Le Huu Huynh Trang”
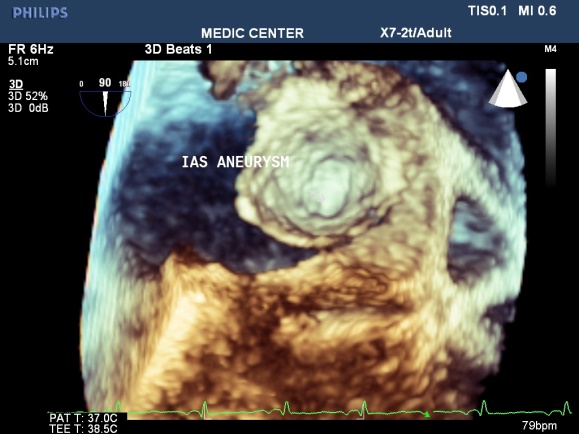
Figure 18. Visualizing ASA by 3D TEE; “Image performed by Nguyen Tuan Vu”
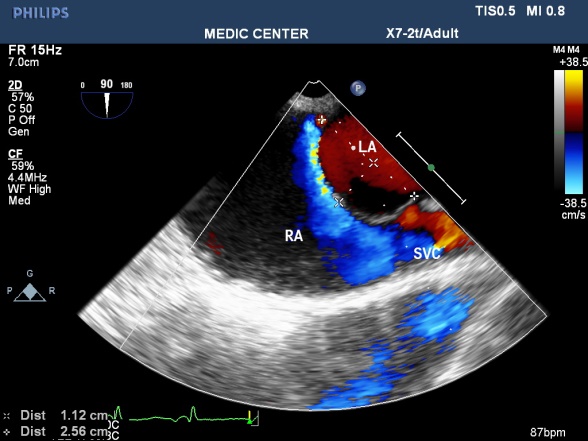
Figure 19. ASA with L-R shunt through PFO; “Image performed by Nguyen Tuan Vu”
Patent Foramen Ovale (PFO)
Foramen ovale is a necessary part of normal circulation in fetus that oxygenated blood from right atrium through it to left atrium to become systemic circulation. It is a communication between the left and right atrium bounded by two membranes representing the septum secundum (right atrial side) and the septum primum (left atrial side). The foramen ovalve is a tunnel between the inferior edge of septum secondum and the superior edge of the septum premium. Natural closure of the foramen ovale usually occurs from 9 to 30 months but that time may take longer. PFO is present in about one-fourth to one-third of adults so PFO is considered a normal variant rather than a pathologic abnormality [29].
The PFO is closed by left-to-right atrial pressure gradient which keeps the two septal membranes together and no shunt left to right is seen. In certain conditions, the right atrial pressure exceeds this of the left side, such as Valsalva maneuver or due to elevation of the right atrial pressure seen with cases of pulmonary hypertension, a right-to-left shunt can be seen through the PFO. Thrombus from venous system or the right heart through PFO to the left atrium and systemic circulation cause embolic events.
PFO accunts for 40-5-% in unexplained stroke [30,31]. So PFO is considered a cause of cardioembolic stroke after exclusion of alternative mechanisms of stroke. The recurrence of stroke due to PFO is about 1-2% annualy. The coexistence of PFO and ASA increases risk of stroke when compare with each condition separately [32].
Our data showed 10 cases of PFO including 9 cases of PFO and a case coexistence of the two conditions (PFO and ASA) (Figure 20).
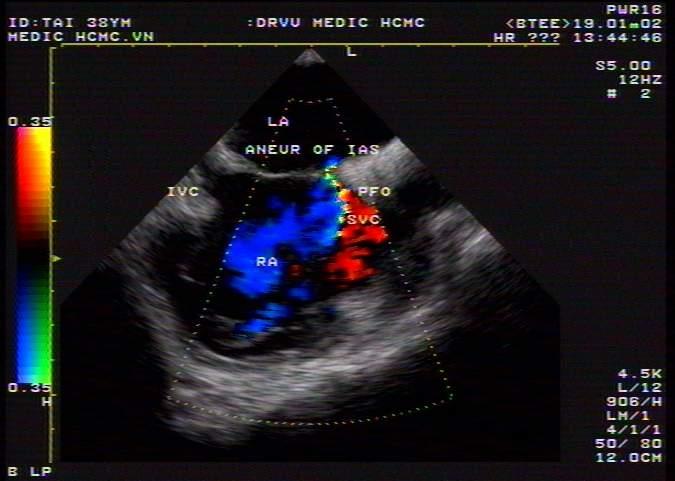
Figure 20. PFO with small L-R shunt; “TEE image performed by Nguyen Tuan Vu”
Mitral valve prolapse
Mitral valve prolapse (MVP) occurs in 2% of the general population.
The risk of thromboembolic complications in MVP occurs when significant thickening of mitral valve, accompanied by LA dilatation, atrial fibrillation [33].
There are 3 cases in our data, all cases are severe mitral regurgitation and LA dilation (Figures 21-23).
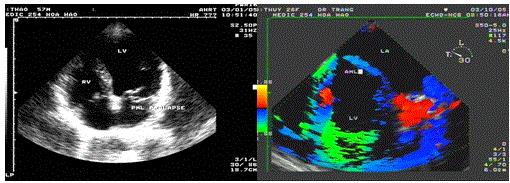
Figure 21. Prolapse of the PML, “TEE with TDI recorded by Le Huu Quynh Trang”
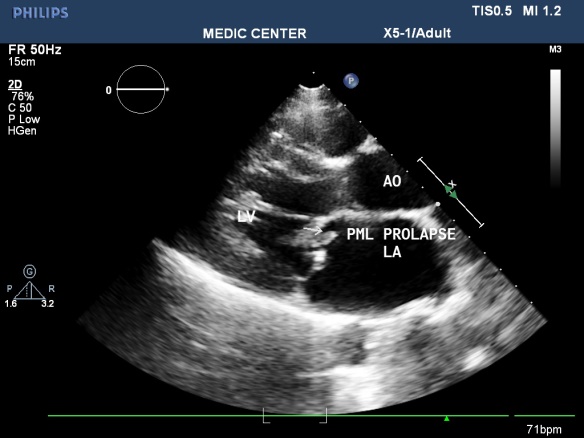
Figure 22. PML prolase due to chordae rupture; “LAX view made by Nguyen Tuan Vu
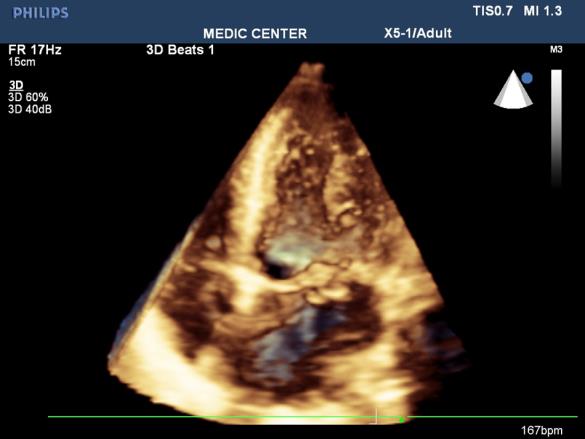
Figure 23. Apcal 4 C view show PML prolapse; 3D TTE performed by Nguyen Tuan Vu
Sick sinus syndrome
Sick sinus syndrome is a common arrhythmia in elderly, cause stroke 5-10% annually. Spontaneos contrast and / or thrombus formation may occur in dilated cardiac chambers. Pace maker may reduce stroke but anticoagulation still is recommended [34].
Beside major risk cardiogenic sources, echocardiography can detect underlying cardiac lesions of cardioembolic strokes. TTE has been shown to be effective in detecting potential cardiac sources at ventricles. Combination of TTE and TEE when necessary obtains more effectiveness in detection of cardiogenic sources in specific cases like LAA thrombus, small LA thrombus, thrombolic events in the thoracic Aorta.
- GBD 2016 Stroke Collaborators (2019) Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 48: 439-458.
- https://www.researchgate.net/publication/226785450_Causes_of_Ischemic_Stroke
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M (2017) Heart disease and stroke statistics – 2017 update: a report from the American Heart Association. Circulation 135: 146-603.
- Carmo JFD, Morelato RL, Pinto HP (2015) Disability after stroke: a systematic review. Fisioter Mov 28: 407-411.
- Le VT, Le TL, Nguyen HH, Dao TX, Nguyen VT, et al. (1999) Strokes in South Vietnam: an epidemiological study. Rev Neurol 155: 137-140.
- Strandberg M, Marttila RJ, Helenius H, Hartiala J (2002) Transoesophagealechocardiography in selecting patients for anticoagulation after ischaemic stroke or transient ischaemic attack. J Neurol Neurosurg Psychiatry 73: 29-33.
- www.emedicine.com/neuro.
- Go AS, Hylek EM, Phillips KA (2001) Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 285: 2370-2375.
- Son MK, Lim N-K, Kim HW, Park H-Y (2017) Risk of ischemic stroke after atrial fibrillation diagnosis: A national sample cohort. PLoS ONE 12: 0179687.
- You John J, Singer Daniel E, Howard Patricia A, Lane Deirdre A, Eckman Mark H, et al. (2012) Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141: 531-575.
- Haft JI, Teichholz LE (2013) High Incidence of Atrial Fibrillation or Flutter in Stroke Patients Who Have the Clinical Risk Factors for Stroke. J Atrial Fibrillation 6: 881.
- Cardiogenic brain embolism (1989) The second report of the Cerebral Embolism Task Force. Arch Neurol 46: 727-743.
- Nakanishi K, Homma S (2016) Role of echocardiography in patients with stroke. J Cardiol 68: 91-99.
- Biase D (2012) Does the Left Atrial Appendage Morphology correlate With the Risk of Stroke in Patients With Atrial Fibrillation? JACC 60: 531-538.
- Arboix A, Alio J (2010) Cardioembolic Stroke: Clinical Features, Specific Cardiac Disorders and Prognosis. Curr Cardiol Rev 6: 150-161.
- Zühlke LJ, Beaton A, Engel ME (2017) Group A streptococcus, acute rheumatic fever and rheumatic heart disease: epidemiology and clinical considerations. Curr Treat Options Cardiovasc Med 19:15.
- Remenyi B, Carapetis J, Wyber R, Taubert K, Mayosi BM (2013) Position Statement of the World Heart Federation on the Prevention and Control of Rheumatic Heart Disease. Nat Rev Cardiol 10: 284-292.
- Reynen K (1995) Cardiac myxoma. N Engl J Med 333: 1610-1617.
- Bayer AS, Bolger AF, Taubert KA (1998) Diagnosis and management of infective endocarditis and complications. Circulation 98: 2936-2948.
- Habib G (2003) Embolic risk in subacute bacterial endocarditis: Determinants and role of transesophageal echocardiography. Curr Cardiol Rep 5:129-136.
- Cheitlin MD, Alpert JS, Amstrong WF (2003) ACC/AHA/ASE 2003 Guideline Update for the Clinical Application of Echocardiography. J Am Coll Cardiol pp:12-14.
- Silver MD, Dorsey JS (1978) Aneurysms of the septum primum in adults. Arch Pathol Lab Med 10: 262-265.
- Go AS, Hylek EM, Phillips KA (2001) Prevalence of diagnosed atrial fibrillation in adults:national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 285: 2370-2375.
- Schneider B, Hanrath P, Vogel P, Meinertz T (1990) Improved morphologic characterization of atrial septal aneurysm by transesophageal echocardiography: relation to cerebrovascular events. J Am Coll Cardiol 16: 1000-1009.
- Silver MD, Dorsey JS (1978) Aneurysms of the septum primum in adults. Arch Pathol Lab Med 102: 62-65.
- Andreas M (1995) Atrial Septal Aneurysm in Adult Patients. Circulation 91: 2785-2792.
- Shinohara T, Kimura T, Yoshizu H, Ohsuzu F (2001) Three-Year Follow-up of an Atrial Septal Aneurysm. Ann Thorac Surg 71: 1672-1673.
- https://www.asecho.org/wp-content/uploads/2016/01/2016_Cardiac-Source-of-Embolism.pdf.
- Tatani SB (2001) Clinical Impact of Transesophageal Echocardiography in Patients with Stroke without Clinical Evidence of Cardiovascular Sources of Emboli. Arq Bras Cardiol 76: 458-461.
- Jaber WA, Klein AL (2001) How often are atrial septal defects associated with thromboembolism? When should they be looked for? Suspect an atrial septal defect if a young patient has a stroke. Cleveland clinic J Med 68: 954-956.
- Cabanes L, Mas JL, Cohen A, Amarenco P, Cabanes PA, et al. (1993) Atrial septal aneurysm and patent foramen ovale as risk factors for cryptogenic stroke in patients less than 55 years of age. A study using transesophageal echocardiography. Stroke 24: 1865-1873.
- Anvierinos JF (2003) Cerebral ischemic events after diagnosis of mitral valve prolapse. Stroke 34: 1339.
- Greenspon AJ (2004) Predictors of stroke in patients paced for sick sinus syndrome. J Am Coll Cardiol 48: 1617-1622.























