Cell cycle arrest in G1 after DNA damage depends on the presence of p53, which is stabilized following DNA damage, leading to its accumulation in cells. We used 3-aminobenzamide (3-AB), a poly(ADP-ribose) polymerase 1 (Parp1) inhibitor, to investigate the influence of Parp1 on p53 levels after gamma radiation of C3D2F1 3T3-a cells with functional p53. After gamma irradiation with 0–8 Gy, the amount of p53 increased in a dose-dependent manner. The expression period of newly expressed p53 showed a tendency of slight extension by 3-AB treatment during the accumulation phase after gamma-irradiation. However, the calculated half-life of p53 was unchanged by 3-AB treatment. These results suggest that Parp1 may be involved in the regulation of the expression of newly expressed p53 after DNA damage.
Mutations in the tumor suppressor p53 are involved in approximately 50% of all human cancers [1,2]. According to Tsuchida et al., roughly 90% of cultured human oral cavity squamous cell carcinoma cells contain p53 mutations [3]. It has been demonstrated that p53, a transcription factor, has important functions after DNA damage, inducing the expression of the cyclin-dependent kinase inhibitor WAF1/CIP1/p21, and delaying or halting S phase and DNA replication [4-6]. G1 arrest after DNA damage is an important checkpoint mechanism in the prevention of tumor formation. Loss of p53 function results in loss of this checkpoint, resulting in progression to S phase without arrest and repair [4,7]. As a result, DNA damage accumulates, and the resultant mutations can lead to tumorigenesis. The key to G1 phase cell cycle arrest is p53 protein [8]. Kastan et al. evaluated the response of cells to gamma-irradiation, and showed that cells with wild-type p53 (p53wt/wt) arrested in either G1 or G2 phase after radiation-induced DNA damage, but in cells harboring dominant-negative mutations or null mutations (p53wt/mut, p53mut/mut, and p53-/-), arrest occurred only in G2 phase [8]. Additionally, when cells are treated with DNA damaging factors such as ultraviolet radiation and 4-nitroquinoline 1-oxide or gamma radiation and actinomycin D, p53 protein levels increase. However, it has been suggested that this is due to post-translational extension of the half-life of p53 [9,10] and is involved in the regulation of DNA strand break re-joining [11,12]. The extended half-life allows p53 to accumulate inside the cell, activating and inhibiting the transcription of gene clusters containing p53-binding sequences, and promoting G1 arrest. Transcriptional targets of p53 include GADD45, MDM-2, WAF1/CIP1/p21, EGFR, and muscle creatine kinase [13-16].
Poly(ADP-ribose) polymerase 1 (Parp1) is an enzyme that catalyzes post-translational poly(ADP-ribosylation). The presence of 3-aminobenzamide (3-AB), a Parp1 inhibitor, suppresses G1 arrest after gamma-irradiation [17,18], as do the Parp inhibitors benzamide and luminol. Conversely, treatment with 3-aminobenzoic acid, an NAD analogue that does not inhibit Parps, does not suppress G1 arrest. Thus, the suppression of G1 arrest is thought to result from the specific inhibition of Parps [19]. In this study, we have investigated the role of Parp1 in the regulation of p53 levels by investigating the influence of 3-AB on p53 protein stabilization after gamma-irradiation-induced DNA damage.
Cell culture
C3D2F1 3T3-a cells, a fibroblast cell line established from 14-day-old embryos of C3H/HeJ and DBA/2J mice, were used in this study. The cells were established and provided by Dr K. Ogawa and his collaborators at Asahikawa Medical University. Cells were seeded at 3×105 cells per 10 cm dish, cultured in DMEM (ICN Biochemical Inc., Costa Mesa, CA, USA) containing 10% fetal bovine serum, and passaged every 3 days. The doubling time was approximately 17 hours. This cell line has been reported to have no mutations between exon 5 and exon 9 of Trp53 [20]. 3-AB was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). 3-AB at 4 mM was added one hour before gamma-irradiation in this study.
Measurement of p53 half-life
C3D2F1 3T3-a cells were gamma-irradiated 2 days after becoming confluent. To detect protein synthesis, [35S]L-methionine was added as 50 mCi/mL one hour following gamma-irradiation. The cells were washed with PBS and harvested, and protein was extracted using high salt RIPA buffer [10 mM Tris-HCl pH 7.4, 0.6 M NaCl, 0.1% SDS, 1% Nonidet P-40, 0.1% sodium deoxycholate, 1 mM EDTA, 10 mg/mL aprotinin (Wako Pure Chemical Industries Inc.), and 1 mM phenylmethyl sulfonyl fluoride]. First, lysates were precleared with mouse IgG (Sigma-Aldrich, St. Louis, MO, U.S.A.) and goat anti-mouse IgG–Sepharose 4B (Zymed, San Francisco, CA, U.S.A.) using proportions of liquid extract with equal amounts of radioactivity. Next, immunoprecipitation was performed using a mouse anti-p53 monoclonal antibody, Ab246 (Merck Millipore, Darmstadt, Germany), using mouse IgG and 4-morpholinepropanesulfonic acid (MOPS, Sigma-Aldrich) as controls. Unbound antibodies were removed by washing 5 times with 1 mL of RIPA buffer containing 0.15 M NaCl. The precipitated protein was separated by 10% SDS polyacrylamide gel electrophoresis, and the gel was dried. Radioactivity was measured using a BAS-2000 Bio-image Analyzer (FUJIFILM Corp., Tokyo).
Newly expressed p53 protein levels one hour after gamma-irradiation
To measure the synthesis of nascent p53, [35S]L-methionine was used as a marker of C3D2F1 3T3-a protein synthesis after gamma-irradiation, and immunoprecipitation was performed using an anti-p53 antibody. The amount of newly expressed p53 increased gamma-ray dose-dependent manner (Figure 1).
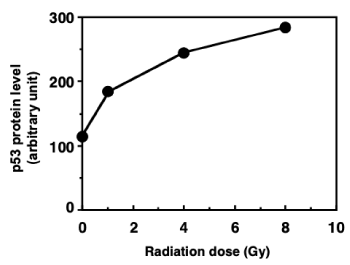
Figure 1. Nascent p53 protein levels one hour after gamma-irradiation in C3D2F1 3T3-a. [35S]L-methionine was added to culture, gamma-irradiated with different doses and immunoprecipitation was carried out using an anti-p53 antibody. Nascent p53 protein levels were calculated with incorporated [35S]L-methionine level at p53 protein bands on the gels
The effect of 3-AB on newly expressed p53 protein accumulation after gamma-irradiation at 2 Gy and 8 Gy
The effect of 3-AB on the amount of newly expressed p53 protein was investigated. One hour after 2 Gy (Figure 2A) and 8 Gy (Figure 2B) of irradiation, the amount of newly expressed p53 protein in the absence of 3-AB was approximately three times and four times higher than control cells, respectively after 2 Gy and 8 Gy irradiation. We noted that in the presence of 3-AB, the slightly augmented increase of newly expressed p53 protein was observed 1 hr after 2 Gy and 8 Gy of gamma-irradiation, although the increase was not statistically significant.
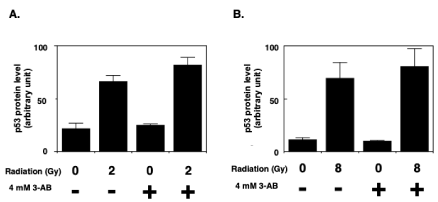
Figure 2. The effect of 3-AB on newly expressed p53 protein accumulation after gamma irradiation. 3-AB was added to cells one hour before gamma irradiation. The p53 protein accumulation level one hour after 2 Gy (A) and 8 Gy (B) irradiation was measured in the absence and presence of 3AB with incorporated [35S]L-methionine level at p53 protein bands on the gels as described in Materials and Methods (n=4)
Influence of 3-AB on the half-life of newly expressed p53 protein after gamma irradiation
To investigate its effect on p53 half-life, 3-AB was added to cells one hour before gamma-irradiation. In the absence of 3-AB, the expression of new p53 peaked 10 minutes after 8 Gy of irradiation and then decreased over time, becoming undetectable by 60 minutes. The expression of new p53 was comparatively delayed in the presence of 3-AB, peaking at 10–20 minutes, and remaining detectable for over 60 minutes (Figure 3A). However, the half-life of p53 was calculated from the linear regression to be approximately 30 minutes and the half-life of p53 was unchanged in the presence of 3-AB (Figure 3B).
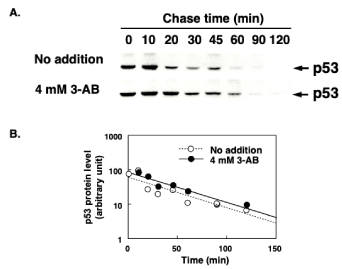
Figure 3. Influence of 3-AB on the half-life of newly expressed p53 protein after gamma irradiation. 3-AB was added to cells one hour before gamma-irradiation. After pulse-labelling of nascent p53 protein, its decay was analyzed with incorporated [35S]L-methionine level at p53 protein bands on the gels. Upper panels show the p53 protein bands detected with 35S radioactivity (A). The lower graph shows the decay profile of nascent p53 protein levels and the effect of 3-AB (B)
The signal transduction mechanisms responsible for G1 arrest after DNA damage rely on p53. We previously reported that G1 arrest after gamma-irradiation is suppressed by inhibitor of Parp, 3-aminobenzamide (3-AB) in C3D2F1 3T3-a cells, which has been reported to have no mutation in exon 5 to exon 9 of the p53 gene [17,18]. We also showed that 3-AB caused suppression of Waf1/Cip1/p21 and Mdm2 induction after gamma-irradiation in C3D2F1 3T3-a cells [21]. In present study, to elucidate the involvement of post-translational poly(ADP-ribosylation) by Parp1 in the regulation of p53 levels, we analyzed p53 stabilization in the cells after DNA damage in the presence of 3-AB. After gamma-irradiation, the effect of 3-AB on p53 stabilization was investigated. Accumulation of p53 after gamma-irradiation has been demonstrated in many different cell types [8,22]. After gamma-irradiation, the p53 protein is stabilized, extending its half-life. There are at least two methods to detect this stabilization, that is determining the total amount of p53 by western blotting or measuring the nascent p53 level using [35S]L-methionine as a metabolic marker. The half-life of p53 is usually quite short (less than 20 minutes) [23], and by detecting the amount of newly expressed p53 protein by immunoprecipitation after gamma-irradiation, the response of p53 after DNA damage can be measured over time. In the present study, we used [35S]L-methionine as a metabolic marker in C3D2F21 3T3-a cells, and investigated changes in the amount of newly expressed p53 protein by immunoprecipitation with an anti-p53 antibody.
Since the C3D2F21 3T3-a cells have no mutations between exons 5–9, the p53-dependent response after DNA damage can be investigated using this cell line [20]. Additionally, C3D2F21 3T3-a cells are an unsynchronized cell population, with approximately 45% of cells in S phase, and cell cycle arrests were observed in C3D2F21 3T3-a cells treated with gamma-irradiation [24]. One hour after radiation, newly expressed p53 protein increased in a radiation dose-dependent manner in this cell lines.
C3D2F1 3T3-a cells were pulse labeled with [35S]L-methionine at 0–1 hour, 1–2 hours, 2–3 hours, and 3–4 hours after gamma-irradiation, and the amount of newly expressed p53 protein was observed. Regardless of the presence or absence of 3-AB, the peak accumulation of newly expressed p53 protein was observed from 0–1 hour (data not shown). The change in the amount of newly expressed p53 protein after gamma-irradiation was therefore investigated one hour after irradiation with a dose of 2 Gy and 8 Gy. By the addition of 3-AB, a slight increase of newly expressed p53 protein was observed 1 hr after gamma-irradiation. Although the results were not statistically significant, the trend in the data suggested that the presence of 3-AB may have affected the G1 arrest process after gamma-irradiation. The results were also consistent with the observation of a slightly increased level of binding activity of p53 to its consensus binding sequences in the presence of 3-AB after gamma-irradiation [21].
Lane et al. reported that 3-AB extended p53 protein stabilization time [25]. We further investigated whether or not the half-life of newly expressed p53 after DNA damage caused by gamma-irradiation was influenced by 3-AB. The p53 level at any point in time will be influenced by the rates of p53 synthesis and degradation. The increase in newly expressed p53 after gamma-irradiation was extended in the presence of 3-AB; however, the half-life was not changed in the presence of 3-AB. In conclusion, the rate of degradation of p53 did not change in the presence of 3-AB. These results possibly suggest that poly(ADP-ribosylation) by Parp1 influences the expression of nascent p53 protein after DNA damage. In the future, to elucidate the role of Parp1 in p53 regulation after DNA damage, mechanisms downstream of p53 should be further investigated. It will be especially useful to determine the influence of Parp inhibitors to the genes on the downstream of p53 dependent pathway.
This work was supported in part by MEXT KAKENHI (15K14416).
The authors have declared that no conflicts of interest exist.
- Hollstein M, Sinransky D, Vogelstein B, Harris CC (1991) p53 mutations in human cancers. Science 253: 49-53.
- Levine AJ, Momand J, Finlay CA (1991) The p53 tumour suppressor gene. Nature 351: 453-456.
- Sakai E, Tsuchida N (1992) Most human squamous cell carcinomas in the oral cavity contain mutated p53 tumor-suppressor genes. Oncogene 7: 927-933.
- El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, et al. (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75: 817-825.
- Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ (1993) The p21 cdk-interacting protein cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75: 805-816.
- Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, et al. (1993) p21 is a universal inhibitor of cyclin kinases. Nature 366: 701-704.
- Dulic V, Kaufmann WK, Wilson SJ, Tisty TD, Lees E, et al. (1994) p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell 76: 1013-1023.
- Kastan MB, Zhan Q, El-Deiry WS, Carrier F, Jacks T, et al. (1992) A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 71: 587-597.
- Tishler RB, Calderwood SK, Coleman CN, Price BD (1993) Increases in sequence specific DNA binding by p53 following treatment with chemotherapeutic and DNA damaging agents. Cancer Res 53: 2212-2216.
- Maltzman W, Czyzyk L (1984) UV irradiation stimulates levels of p53 cellular tumor antigen in nontransformed mouse cells. Mol Cell Biol 4: 1689-1694.
- Oberosler P, Hloch P, Ramsperger U, Stahl H (1993) p53-catalyzed annealing of complementary single-stranded nucleic acids. EMBO J 12: 2389-2396.
- Bakalkin G, Yakovleva T, Selivanova G, Magnusson KP, Szekely L, et al. (1994) p53 binds single-stranded DNA ends and catalyzes DNA renaturation and strand transfer. Proc Natl Acad Sci USA 91: 413-417.
- Price BD, Park SJ (1994) DNA damage increases the levels of MDM2 messenger RNA in wtp53 human cells. Cancer Res 54: 896-899.
- Deb SP, Munoz RM, Brown DR, Subler MA, Deb S (1994) Wild-type human p53 activates the human epidermal growth factor receptor promoter. Oncogene 9: 1341-1349.
- Zambetti GP, Bargonetti J, Walker K, Prives C, Levine AJ (1992) Wild-type p53 mediates positive regulation of gene expression through a spesific DNA sequence element. Genes Dev 6: 1143-1152.
- El-Deiry WS, Harper JW, O’Connor PM, Velculescu VE, Canman CE, et al. (1994) WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res 54: 1169-1174.
- Masutani M, Nozaki T, Wakabayashi K, Sugimura T (1995) Role of poly(ADP-ribose) polymerase in cell-cycle checkpoint mechanisms following gamma-irradiation. Biochimie 77 :462-465.
- Nozaki T, Nakamoto K, Myat AB, Sasaki Y, Imamichi S, et al. (2020) Effects of 3-aminobenzamide on cell cycle arrest after gamma-irradiation. J Transl Sci 6: 1-6.
- Nozaki T, Masutani M (2018) p53-dependent cell cycle checkpoint after DNA damage and its relevance to PARP1. Res Rev Insights 2(2): 1-5.
- Tokumitsu M, Kadohama T, Ogawa K (1994) Infrequent loss of heterozygosity and mutation of the p53 gene in immortal and transformed mouse embryo fibroblasts. Mol Carcinog 10: 52-57.
- Nozaki T, Masutani M (2018) Involvement of Parp1 in the downstream of p53 dependent signaling pathway induced after gamma-irradiation. J Transl Sci 5: 1- 5.
- Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW (1991) Participation of p53 protein in the cellular response to DNA damage. Cancer Res 51: 6304-6311.
- Reihsaus E, Kohler M, Kraiss S, Oren M, Montenarh M (1990) Regulation of the level of the oncoprotein p53 in non-transformed and transformed cells. Oncogene 5(1): 137-145.
- Nozaki T, Masutani M, Akagawa T, Sugimura T, Esumi H (1994) Suppression of G1 arrest and enhancement of G2 arrest by inhibitors of poly(ADP-ribose) polymerase: possible involvement of poly(ADP-ribosyl)ation in cell cycle arrest following gamma-irradiation. Jpn J Cancer Res 85: 1094-1098.
- Lu X, Lane DP (1993) Differential induction of transcriptionally active p53 following UV or ionizing radiation: defects in chromosome instability syndromes? Cell 75: 765-778.



