High resting heart rate (HR) is independently associated with cardiovascular disease risk. Few studies took into consideration the possible effects of HR on memory. Therefore, memory with interference (MI) at 10 (MI10) and at 30 (MI30) seconds was chosen to test this hypothesis and the role of resting HR on a MI in hypertensive subjects was analysed. MI was chosen because is strictly connected with everyday life.
Among 832 hypertensive subjects aged 18-88 years living in North East Italy recruited in the frame of the Growing Old with Less Disease Enhancing Neurofunctions (GOLDEN) study, we performed an association analysis, accounting for potential confounders, to identify the possible determinants of MI.
Both in univariate and multivariate regression analysis, MI10 and MI30 were indirectly associated to basal HR: the higher the HR, the lower the MI. Years of schooling was associated directly and age inversely to both MI10 and MI30. A progressive trend towards reduction of the score was particularly evident for MI30, with two evident cutoff values (equal to 65 and to 73 bpm, respectively). In the case of MI10, 60 bpm was the upper limit of the best performance. Furthermore, being in the first three quartiles rather than in the 4th quartile of MI30 was significantly lower.
High HR should be counted among the risk factors or indicators of low memory. The inverse association between high HR and low memory is not ineluctable as it can be prevented by education.
Abbreviations
BP: arterial blood pressure; LDL: low-density-lipoprotein; HT: arterial hypertension
heart rate, hypertension, cognition, memory
Many papers from different research groups have shown that high heart rate (HR) is independently associated with cardiovascular disease risk. Research on this field is centred on the cardiovascular system, high HR leading to worse mortality and morbidity and being also associated to metabolic risk [1-9].
To our knowledge, the question of whether HR influences cognition has been scarcely investigated in hypertensive subjects. A limited number of papers in this field took into account HR variability but not HR per se [10]. In different analyses, higher resting HR variability predicted enhanced memory inhibition, and lower HR variability was associated with higher risk of future functional decline in older adults [11,12].
In the present epidemiological analysis, we took into consideration the possible effect of basal (resting) HR on memory. Memory with interference (MI) was chosen to test this hypothesis. The tests of MI at 10 and 30 seconds (MI10 and MI30) are of special relevance because they reproduce in laboratory the conditions of everyday life, where important inputs have to be understood and durably remembered while interfering stimuli not directly correlated with the main information arrive from usual interfering activities [13]. MI is how much more similar, in laboratory and in epidemiology, to this natural situation. Stable memorization of information despite undercurrent distractions is of paramount importance because it contributes to build the autobiographic memory which is the basis of the egoic (subjective) consciousness and of the feeling of self. Many factors influence MI (mainly age and education), but basal HR has never been considered one of its determinants.
Cohort: Eight hundred and thirty-two hypertensive subjects (427 men and 405 women) aged 18-88 years (57.4±12.2 years), living in North East Italy in a semi-rural area of about 504 km2 in the Leogra and Illasi valleys and sharing a homogeneous lifestyle, were studied in 2012-2018 in the frame of the Growing Old with Less Disease Enhancing Neurofunctions (GOLDEN) study [14-17]. The protocol of the study was extensively described elsewhere and is here briefly summarized [14-17].
All subjects aged ≥18 years living in that area were contacted by letter and then, when necessary, by phone from the registry office lists. The attendance rate was 73% and the subjects analyzed in the present paper represent the first 832 hypertensive recruited. No significant social or demographic differences were detected between the subjects included in the study and the other subjects belonging to the same population object of the ongoing study (data not shown).
The investigation conformed to the Declaration of Helsinki and was approved by the local Ethics Committees of the University of Padua and Verona (Italy). Each subject was previously and personally informed about the aim and the meaning of the study and was free to ask all the questions they felt necessary to have a full comprehension of it. All the participants gave a valid written informed consent and signed a form approved by the Ethics Committee according to Italian law 675/1996 and to the law of the Veneto Region 34/2007.
General items: At a clinical visit, all subjects underwent a detailed Rose’s questionnaire concerning lifestyle, smoking and drinking habits, physical activity, quality of life, medication, and personal and familial medical history. Education was defined as number of years of schooling based on the highest educational qualification achieved. Height (in m) and weight (in kg) were recorded without shoes with the subjects wearing light indoor clothing. Body mass index was calculated in (kg/m2) as body weight divided by squared height. HR was taken from basal electrocardiographic RR intervals in a range of 15-min rest. Systolic and diastolic (Korotkoff phase 5) blood pressure was taken in triplicate at 10-min intervals in the supine position by trained doctors using an Omron 705 IT device (Omron Europe, Hoofddorp, Netheralnds). To minimize white-coat effects, the average of the last two measurements (in mmHg) was taken into consideration. The label of arterial hypertension required systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or history of hypertension or appropriate antihypertensive treatment or hospital discharge with diagnosis-related group (DRG) 401–404 or 40200–40290 or 40300–40391 [18]. Treatment of hypertension was ascertained by hospital discharge files, general practitioners’ files and asking subjects and double-checked.
A 138-item food frequency detailed questionnaire previously validated for the Mediterranean diet was administered at the initial screening. The reported frequencies of food intake per week were converted to number of intakes per day and multiplied by the weight of the portion size indicated. Each food was then resolved into its chemical components according to composition tables conceived for Italian food, where data were expressed as percent of food actually consumed after eliminating the scrap. We therefore calculated fish, lipidic and glucidic intake (g/day). This method was already used and validated by our group. Daily caffeine intake was calculated from the formula: caffeinemmol/day = [coffeecups/day x 0.412 + teacups/day x 0.214 + coladrinks/day x 0.082 + chocolateportions/day x 0.081] and ethanol intake from: ethanolg/day = [(wineml/day x 0.12 + beerml/day x 0.05 + aperitifsml/day x 0.11+ liquorsml/day x 0.40) x 0.80] [19-20].
Blood glucose, serum uric acid and low-density-lipoprotein (LDL) cholesterol were measured at fast by automated standard methods.
MI10 and MI30: Participants were seen by a neuropsychologist at an ad hoc hospital unit. They were requested to recall a consonant trigram after an interval delay during which they had to count backward starting from a random number suggested by the examiner immediately after the trigram [13]. More in details, each subject was individually invited to observe three cardboards with 3 alphabetic letters each. Subject had to read the letter aloud and try to memorize them. Then the cardboards were covered, and the subject was asked to count by twos for 10 seconds (MI10) starting from a number randomly chosen by the researcher. After these 10 seconds, subject had to repeat the trigrams, and the number of correct letters was recorded and used as score. If this test was completed with a score >0, it was repeated with different trigrams asking the participant to count by twos for 30 seconds (MI30) and to repeat the trigrams, recording the number of letters correctly repeated. The aim of those two tests is to ascertain the ability of subjects to remember short-term information while they were distracted immediately after the memorization by external events of different duration, as it happens in everyday life [13].
Statistical analysis: For database management and statistical analysis, the Statistical Analysis System (SAS) software version 9.3 (SAS Institute, Cary, USA) was used. The null hypothesis was always rejected when p value was <0.05. A priori power analysis showed that 200 subjects per cell were sufficient to show effects (power 0.90, test level 0.10 for β error and 0.05 for α error), assuming a putative difference of 1point score in MI10 or MI30 between the highest and lowest HR. This difference was chosen a priori based on preliminary tests of our laboratory, as no data on the effects of HR on MI in the general population exist. Our 832 subjects also appeared adequate after stratification into quartiles.
Continuous variables were expressed as mean and standard deviation and compared with analysis of variance and the post hoc Bonferroni's correction. Categorical variables were expressed as percent rates and compared with the Pearson's χ2 test.
Two multivariate regression analysis (REGLIN), having MI10 and MI30 respectively as dependent variables, were used to identify the possible determinants of MI. Sex, age, schooling, systolic and diastolic blood pressure, resting HR, ethanol and caffeine intake, cigarettes, blood glucose, serum LDL cholesterol and uric acid, fish, lipids and glucose intake, and treatment of hypertension, logarithmized when proper, were considered as possible confounders.
Increasing quartiles of HR were created. Analysis of covariance adjusted for the above-mentioned confounders was first used to check for differences in continuous variables between quartiles of HR. To this purpose, p for trend were shown.
The first three quartile of HR were also cumulated together and played in a multiple regression having MI10 and MI30 respectively as dependent variable adjusted for the same confounders, in order to calculate the hazard ratio to be in the fourth quartile.
In sensitivity analysis, to explore the reverse causality, HR was used in REGLIN as dependent variable and MI10 e MI30, respectively as independent covariables adjusted for the same confounders.
Descriptive statistics: The general characteristics of the cohort are summarized in Table 1, also showing stratification by quartiles of HR. Across quartile of HR, there is a progressive increase of systolic and diastolic blood pressure and serum blood glucose. MI10 score was in average 5.43 ± 2.79 (range 0-9, 95%CI 5.24-6-62) and MI30 score 3.85 ± 3.25 (range 0-9, 95%CI 3.63-4-07) [Table 1].
Table 1. General characteristics of the study population (n=832), also showing stratification by quartiles of resting heart rate. Number of subjects in brackets. Differences between groups analyzed by analysis of variance followed by Bonferroni’s tests. §Average of two blood pressure readings obtained at clinic. *The body mass index is weight in kilograms divided by the square of the height in meters. ∆To convert values for caffeine intake to milligrams per day, multiple by 194.21
|
|
Quartiles of resting heart rate
|
|
|
Whole cohort
(n=832) |
I quartile
(n=208) |
II quartile
(n=208) |
III quartile
(n=208) |
IV quartile
(n=208) |
p-value
for trend |
Continuous variables |
|
|
|
|
|
|
Resting heart rate (bpm) |
67.1 ± 10.6 |
55.1 ± 4.4 |
62.6 ± 1.8 |
69.8 ± 2.2 |
81.0 ± 7.6 |
<0.0001 |
Age (years) |
57.4 ± 12.3 |
62.4 ± 15.2 |
61.6 ± 13.0 |
61.9 ± 15.8 |
62.1 ± 15.5 |
0.96 |
Schooling (years) |
8.3 ± 3.5 |
8.5 ± 3.9 |
7.9 ± 3.3 |
8.3 ± 3.3 |
7.9 ± 3.2 |
0.18 |
Systolic BP (mmHg) § |
153.6 ± 19.9 |
151.5 ± 17.8 |
151.3 ± 20.1 |
153.8 ± 20.5 |
157.7 ± 20.5 |
<0.01 |
Diastolic BP (mmHg) § |
89.7 ± 9.2 |
87.4 ± 8.2 |
88.9 ± 9.5 |
91.1 ± 9.2 |
91.3 ± 9.3 |
<0.0001 |
Body mass index (kg/m2)* |
27.3 ± 4.4 |
27.0 ± 4.0 |
27.5 ± 4.7 |
27.6 ± 4.9 |
26.8 ± 3.8 |
0.16 |
Cigarettes/day in smokers |
12.3 [10.3-13.6] |
11.6 [8.6-14.5] |
10.5 [7.0-14.2] |
13.6 [10.4-16.8] |
12.2 [8.4-16.0] |
0.58 |
Ethanol intake (g/day) |
47.2 [44.2-50.3] |
47.9 [41.7-54.1] |
47.4 [41.9-53.0] |
45.6 [39.8-51.4] |
47.9 [41.0-54.7] |
0.95 |
Caffeine intake (mmol/day)∆ |
1.00 ± 0.68 |
1.02 ± 0.70 |
1.04 ± 0.66 |
0.90 ± 0.63 |
1.03 ± 0.70 |
0.11 |
Serum glucose (mmol/l) |
5.41 ± 1.03 |
5.23 ± 0.78 |
5.40 ± 1.07 |
5.44 ± 1.03 |
5.58 ± 1.17 |
<0.01 |
Serum uric acid (µmol/l) |
305.2 ± 78.0 |
304.4 ± 72.8 |
307.6 ± 79.9 |
297.7 ± 81.3 |
311.2 ± 80.7 |
0.33 |
LDL serum cholesterol (mmol/l) |
3.71 ± 0.93 |
3.67 ± 0.90 |
3.79 ± 0.92 |
3.68 ± 0.93 |
3.72 ± 0.97 |
0.57 |
Fish intake (g/week) |
100.4 ± 115.3 |
105.3 ± 109.2 |
109.0 ± 122.4 |
98.5 ± 132.0 |
89.0 ± 94.8 |
0.31 |
Glucose intake (g/week) |
906.7 ± 380.7 |
914.1 ± 393.5 |
897.0 ± 408.0 |
905.4 ± 368.1 |
910.3 ± 354.6 |
0.99 |
Lipid intake (g/week) |
548.1 ± 256.2 |
575.7 ± 261.3 |
554.8 ± 246.7 |
525.5 ± 252.5 |
536.5 ± 263.0 |
0.26 |
Categorical variables |
|
|
|
|
|
|
Males, n (%) |
427 (51.3) |
125 (60.1) |
116 (56.0) |
98 (47.1) |
88 (42.3) |
<0.0001 |
Treatment of HT, n(%) |
367 (44.2) |
84 (40.4) |
91 (44.0) |
97 (46.6) |
95 (45.7) |
0.59 |
Smokers, n(%) |
98 (11.2) |
26 (12.5) |
26 (12.5) |
26 (12.5) |
20 (9.6) |
0.73 |
Drinkers, n(%) |
525 (63.2) |
140 (67.3) |
143 (69.1) |
120 (57.7) |
122 (58.7) |
0.03 |
Diabetics, n (%) |
50 (6.0) |
6 (2.9) |
11 (5.3) |
15 (7.2) |
18 (8.7) |
0.08 |
Multivariate analysis of MI as continuous variable: Multivariate REGLIN showed that both MI10 and MI30 were inversely associated to HR (Table 2): the lower the HR, the greater the MI.
Years of schooling were associated directly and age inversely to both MI10 and MI30 (Table 2), although unable to nullify the HR ↔ MI relationship. No other determinants or confounders were identified [Table 2].
Table 2.Determinants of the tests of memory with interference at 10 seconds and at 30 seconds. MI30 and MI30, respectively, are the dependent variables. Multivariate regression analysis adjusted for plausible biological confounders
Variables |
Coefficient
(standard error) |
Standard coefficient |
p-value
|
Test of memory with interference at 10 seconds |
|
Determinants |
Resting heart rate (bpm) |
-1.7517 (0.6651) |
-0.0949 |
<0.01 |
Age (years) |
-2.9522 (0.5713) |
-2.2640 |
<0.0001 |
Schooling (years) |
2.2595 (0.3204) |
0.3213 |
<0.0001 |
|
Possible confounders |
Sex (0=women, 1= men) |
-0.4055 (0.2590) |
-0.0697 |
0.12 |
Systolic BP (mmHg) |
-1.7567 (0.9799) |
-0.0820 |
0.07 |
Diastolic BP (mmHg) |
1.6205 (1.1245) |
0.0605 |
0.15 |
Serum LDL-cholesterol (mmol/l) |
-0.2014 (0.4403) |
-0.0171 |
0.65 |
Blood glucose (mmol/l) |
-0.3455 (0.6655) |
-0.0203 |
0.61 |
Serum uric acid (µmol/l) |
0.3535 (0.4585) |
0.0310 |
0.44 |
Caffeine intake (mmol/day) |
0.0011 (0.0008) |
0.0471 |
0.19 |
Ethanol intake (g/day) |
0.0010 (0.0030) |
0.0131 |
0.74 |
Fish intake (g/week) |
-0.0005 (0.0008) |
-0.0215 |
0.55 |
Lipid intake (g/week) |
0.0002 (0.0004) |
0.0144 |
0.70 |
Glucose intake (g/week) |
0.0005 (0.0003) |
0.0612 |
0.12 |
Treatment HT (0=no, 1=yes) |
-0.1987 (0.2305) |
-0.0341 |
0.39 |
Smoking (cigarettes/day) |
0.0084 (0.0225) |
0.0134 |
0.71 |
Test of memory with interference at 30 seconds |
|
Determinants |
Resting heart rate (bpm) |
-1.7241 (0.6880) |
-0.0819 |
0.012 |
Age (years) |
-4.7657 (0.5824) |
-0.3798 |
<0.0001 |
Schooling (years) |
2.4628 (0.3372) |
0.3104 |
<0.0001 |
|
Possible confounders |
Sex (0=women, 1= men) |
-0.0890 (0.2702) |
-0.0135 |
-0.74 |
Systolic BP (mmHg) |
-1.8652 (1.0155) |
-0.0764 |
0.07 |
Diastolic BP (mmHg) |
0.6606 (1.1664) |
0.0215 |
0.57 |
Serum LDL-cholesterol (mmol/l) |
-0.5182 (0.4473) |
-0.0391 |
0.25 |
Blood glucose (mmol/l) |
-0.7352 (0.6778) |
-0.0383 |
0.28 |
Serum uric acid (µmol/l) |
-0.0692 (0.4746 |
-0.0054 |
0.28 |
Caffeine intake (mmol/day) |
0.0004 (0.0008) |
0.0144 |
0.66 |
Ethanol intake (g/day) |
-0.0018 (0.0031) |
-0.0211 |
0.56 |
Fish intake (g/week) |
-0.0002 (0.0008) |
-0.0057 |
0.86 |
Lipid intake (g/week) |
0.0003 (0.0004) |
0.0268 |
0.43 |
Glucose intake (g/week) |
-0.0003 (0.0003) |
-0.0324 |
0.38 |
Treatment of HT (0=no, 1=yes) |
0.0892 (0.2351) |
0.0134 |
0.70 |
Smoking (cigarettes/day) |
-0.0004 (0.0008) |
-0.0539 |
0.10 |
In sensitivity analysis, inversing the REGLIN model (i.e. using MI10 or MI30 as dependent variables and HR as an independent covariable), memory did not result to be a determinant of HR: in fact, HR was only determined by sex (coefficient -0.050, standard error, SE, 0.017, standard coefficient 0.588, p=0.003), by diastolic blood pressure (coefficient 0.295, SE 0.073, standard coefficient 0.693 p<0.001) and by serum blood glucose (coefficient 0.110, SE 0.043, standard coefficient 0.786, p<0.01), while MI30 and MI10 were rejected from the model.
Univariate and multivariate analysis of quartiles of MI: After dividing HR into 4 quartiles of 208 subjects each and comparing them with analysis of covariance adjusted for schooling and age, both MI10 and MI30 were significantly greater in the first quartile than in the fourth (Figure 1).
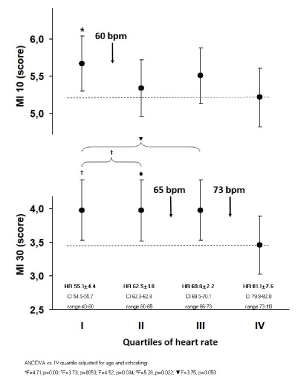
Figure 1. Scores of memories with interference at 10 seconds (MI10) and at 30 seconds (MI30) by quartiles of heart rate (n=208 each). ANCOVA vs. IV quartile adjusted for age and schooling: *p=0.03 (F=4.71), †p=0.005 (F=3.73), ‡p=0.022 (F=5.28), qp=.0053 (F=3.75)
MI30 was also greater in the first two quartiles cumulated together than in the forth, while the difference between the first three quartiles cumulated together and the forth was near to statistical significance although not sufficient to exclude the null hypothesis (p=0.053) (Figure 1).
For MI30, the between-quartile boundaries were 60 bpm from the I and the II, 65 bpm from the II and the III, and 73 bpm from the III and the IV. It appeared that 60 bpm was a clear cut-off value associated to significant change in MI10 (Figure 1): when this cut-off was used as a factor in analysis of covariance, it definitely divided lower and higher values of MI10 scores (F=5.79, p=0.016). Analogously, 73 bpm clearly divided lower and higher values of MI30 scores (F=4.17, p=0.041).
When the 4th quartile was used as independent variable in adjusted multivariate REGLIN, the hazard ratio of being in the the first three quartiles rather than in the IV was 0.560 (95% confidence intervals 0.996-0.358; p=0.047) for MI30, while the results for MI10 were not significant (hazard ratio 0.748, 95% confidence intervals 0.485-1.542, p=0.2).
HR as an inverse determinant of cognition: Both the univariate and multivariate analyses demonstrate that MI is indirectly associated to basal HR: the higher the HR, the lower the MI. A progressive trend towards reduction of the score was particular evident for MI30, with values of HR equal to 65 and 73 bpm as cutoff values between quartiles. In the case of MI10, 60 bpm was the upper limit of the best performance. Furthermore, at multivariate REGLIN, the risk of being in the first three rather than in the IV quartile of MI30 was significantly lower. The symmetrical hypothesis that MI inversely determines HR was excluded by the fact that no significant association was found between these two items when HR was used as dependent variable.
An attempt to explain the association: The data shown herein simply indicate that, as regards everyday memory, it is better to have lower than higher HR values. The reasons of this evidence go beyond the instruments available to epidemiologists and can only be object of speculation. High blood pressure (a factor that notoriously tends to decrease the cognitive abilities) was not accepted in the multivariate models and therefore cannot be singled out to explain the phenomenon. A link between HR and memory in the frame of an echogenetic context cannot be excluded but is far to be demonstrated [21]. The hypothesis that high-HR subjects are sympathetically hyperstimulated and therefore less able to focus attention on stimuli and concepts precluding optimal memorization must be rejected, as it is known that arousal experienced during the exposure to an event able to enhance the release of norephinephrine and epinephrine (two agonists influencing the amygdala and the hippocampus during encoding and consolidation) leads to improved memory [22]. Activation of β-adrenergic receptors during consolidation even helps memory formation and later remembering and norepinephrine is necessary for reconsolidation of certain types of the degraded memory [23-25]. Finally, at a population level, overweight subjects with hyper-stimulated sympathetic drive have shown enhanced cognitive performance [26]. On the contrary, what leads to low HR, such as isotonic exercise and sleep, tends to favour memory, while sleep deprivation reduces cognitive abilities [27-32]. Experimental studies are mandatory in order to clarify the mechanism underlying the HR ↔ cognition relationship put in evidence in the present pilot epidemiological analysis.
Consequences of our results: One consequence of the results of the present study is that high HR should be counted among the risk factors or indicators of low memory, a circumstance that could be of importance, for instance, in hiring workers for whom memory can play an important role.
Another consideration is that the inverse, unwanted association between high HR and low memory is not ineluctable, as it can be prevented by education. In fact, years of schooling potently enter directly and independently the models having MI10 and MI30 as dependent variables, strongly reducing the negative effect of high HR. Schooling persisted for years is therefore the best way to counteract the negative effect of tachycardia on memory and the deleterious effects of age in increasing this association.
Is an intervention plausible? HR deceleration is triggered and controlled by subcortical structures (amygdala and brainstem)33. Having low HR is convenient from a cognitive point of view, but the question on whether reducing pharmacologically HR leads to best memory is of course unanswered. If so, for the reasons exposed above, β-blockers (reducing the sympathetic drive) would not be a good choice [22]. No information at all is available in this respect as regards non-adrenergic reduction of HR via ivabradine. Non-pharmacological ways to reduce HR are to reduce stress (which increases HR) and practicing endurance isotonic exercise, which in fact improves cognitive functions and memory in particular [27,28,34-37]. Another way is sleep, which notoriously reduces HR and, as demonstrated, consolidates immediate emotional meaning by enhancing automatic responses and promotes top-down control of emotional responses by strengthening respective neocortical representations and increases or preserves the emotional tone associated with memories [29-31]. A simple nap after a learning session can improve assimilation of information and memory [29].
This research was funded by a grant from the Italian Ministry of Health (Research “Growing Old with Less Disease Enhancing Neurofunctions, GOLDEN”, CUP H71J11001020001) awarded to Prof. Casiglia.
The Authors declares that there is no conflict of interest
- Palatini P, Saladini F, Mos L, Fania C, Mazzer A, et al. (2018) Low night-time heart rate is longitudinally associated with lower augmentation index and central systolic blood pressure in hypertension. Eur J Appl Physiol 118: 543-550.
- Palatini P, Julius S (2014) Resting heart rate: an independent predictor of congestive heart failure. J Am Coll Cardiol 64: 421-422. [Crossref]
- Palatini P (2017) Heart Rate Reduction and Cardiovascular Outcome in Hypertension. J Am Coll Cardiol 69: 1099-1100. [Crossref]
- Palatini P, Reboldi G, Beilin LJ, Casiglia E, Eguchi K, et al. (2017) Masked tachycardia. A predictor of adverse outcome in hypertension. J Hypertens 35: 487-492. [Crossref]
- Palatini P1, Reboldi G, Beilin LJ, Eguchi K, Imai Y, et al. (2013) Predictive value of night-time heart rate for cardiovascular events in hypertension. The ABP-International study. Int J Cardiol 168: 1490-1495. [Crossref]
- Palatini P, Agabiti Rosei E, Casiglia E, Chalmers J, Ferrari R, et al. (2016) Management of the hypertensive patient with elevated heart rate: Statement of the Second Consensus Conference endorsed by the European Society of Hypertension. J Hypertens 34: 813-821.
- Palatini P, Casiglia E, Julius S, Pessina AC (1999) High heart rate: a risk factor for cardiovascular death in elderly men. Arch Intern Med 159: 585-592. [Crossref]
- Palatini P (2013) Heart rate and the cardiometabolic risk. Curr Hypertens Rep 15: 253-259. [Crossref]
- Palatini P, Casiglia E, Pauletto P, Staessen J, Kaciroti N, et al. (1997) Relationship of tachycardia with high blood pressure and metabolic abnormalities: a study with mixture analysis in three populations. Hypertension 30: 1267-1273.
- Liu J, Wei W, Kuang H, Zhao F, Tsien JZ (2013) Changes in heart rate variability are associated with expression of short-term and long-term contextual and cued fear memories. PLoS One 8: e63590. [Crossref]
- Gillie BL, Vasey MW, Thayer JF (2014) Heart rate variability predicts control over memory retrieval. Psychol Sci 25:458-465.
- Ogliari G, Mahinrad S, Stott DJ, Jukema JW, Mooijaart SP, et al. (2015) Resting heart rate, heart rate variability and functional decline in old age. CMAJ 187: E442-442E449. [Crossref]
- Mondini S, Mapelli D, Vestri A, Bisiacchi PS (2003) Esame neuropsicologico breve: una batteria di test per lo screening neuropsicologico. Milano: Cortina.
- Casiglia E, Giordano N, Tikhonoff V, Boschetti G, Mazza A (2013) Cognitive functions across the GNB3 C825T polymorphism in an elderly Italian population. Neurol Res Int 597034.
- Casiglia E, Basso G, Guglielmi F, Martini B, Mazza A, et al. (2005) German origin clusters for high cardiovascular risk in an Italian enclave. Int Heart J 46: 489-500. [Crossref]
- Casiglia E, Tikhonoff V, Boschetti G, Bascelli A, Saugo M, et al. (2012) The C825T GNB3 polymorphism, independent of blood pressure, predicts cerebrovascular risk at a population level. Am J Hypertens 25:451-457.
- Casiglia E, Tikhonoff V, Caffi S, Boschetti G, Grasselli C, et al. (2013) High dietary fiber intake prevents stroke at a population level. Clin Nutr 32: 811-818. [Crossref]
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, et al. (2013) ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Europ Heart J 34: 2159-2219.
- Casiglia E, Tikhonoff V, Albertini F, Gasparotti F, Mazza A, et al. (2018) Caffeine intake reduces incident atrial fibrillation at a population level. Eur J Prev Cardiol 25: 1055-1062.
- Casiglia E, Paleari CD, Petucco S, Bongiovì S, Colangeli G, et al. (1992) Haemodynamic effects of coffee and purified caffeine in normal volunteers: a placebo-controlled clinical study. J Hum Hypertens 6: 95-99.
- Stolarz K, Staessen JA, Kawecka-Jaszcz K, Brand E, Bianchi G, et al. (2004) Genetic variation in CYP11B2 and AT1R influences heart rate variability conditional on sodium excretion. Hypertension 44: 156-162.
- Rimmele U, Lackovic SF, Tobe RH, Leventhal BL, et al. (2016) Beta-adrenergic Blockade at Memory Encoding, but Not Retrieval, Decreases the Subjective Sense of Recollection. J Cogn Neurosci 28: 895-907. [Crossref]
- Barsegyan A, McGaugh JL, Roozendaal B (2014) Noradrenergic activation of the basolateral amygdala modulates the consolidation of object-in-context recognition memory. Front Behav Neurosci 8:160.
- Roozendaal B, Castello NA, Vedana G, Barsegyan A, McGaugh JL (2008) Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiol Learn Memory 90: 576-579.
- Littel M, Kenemans JL, Baas JMP, Logemann HNA, Rijken N, et al. (2017) The effects of ß-adrenergic blockade on the degrading effects of eye movements on negative autobiographical memories. Biol Psychiatry 82: 587-593.
- Tikhonoff V, Casiglia E, Guidotti F, Giordano N, Martini B, et al. (2015) Body fat and the cognitive pattern: a population-based study. Obesity 23: 1502-1510.
- Most SB, Kennedy BL, Petras EA (2017) Evidence for improved memory from 5 minutes of immediate, post-encoding exercise among women. Cogn Res Princ Implic 2: 33.
- McNerney MW, Radvansky GA (2015) Mind racing: the influence of exercise on long-term memory consolidation. Memory 23: 1140-1151.
- Bolinger E, Born J, Zinke K (2018) Sleep divergently affects cognitive and automatic emotional response in children. Neuropsychologia 117: 84-91.
- Wagner U, Fischer S, Born J (2002) Changes in emotional responses to aversive pictures across periods rich in slow-wave sleep versus rapid eye movement sleep. Psychosom Med 64: 627-634.
- Werner GG, Schabus M, Blechert J, Kolodyazhniy V, Wilhelm FH (2015) Pre-to postsleep change in psychophysiological reactivity to emotional films: late-night REM sleep is associated with attenuated emotional processing. Psychophysiology 52: 813-825.
- Patrick Y, Lee A, Raha O, Pillai K, Gupta S, et al. (2017) Effects of sleep deprivation on cognitive and physical performance in university students. Sleep Biol Rhythms 15: 217-225.
- Öhman A, Hamm A, Hugdahl K (2000) Cognition and the autonomic nervous system: orienting, anticipation, and conditioning. In: Cacioppo JT, Tassinary LG, Berntson GG (eds). Handbook of Psychophysiology, 2nd ed. Cambridge: Cambridge University Press 533-575.
- Delgado-Moreno R, Robles-Pérez JJ, Clemente-Suárez VJ (2017) Combat stress decreases memory of warfighters in action. J Med Syst 41: 124.
- Barry A, Cronin O, Ryan AM, Sweeney B, O'Toole O, et al. (2018) Impact of short-term cycle ergometer training on quality of life, cognition and depressive symptomatology in multiple sclerosis patients: a pilot study. Neurol Sci 39:461-469.
- Mahinrad S, Jukema JW, van Heemst D, Macfarlane PW, Clark EN, et al. (2016) 10-Second heart rate variability and cognitive function in old age. Neurology 86: 1120-1127. [Crossref]
- Lin F, Heffner KL, Ren P, Tadin D (2017) A Role of the Parasympathetic Nervous System in Cognitive Training. Curr Alzheimer Res 14: 784-789. [Crossref]

