Objective: Problems faced by pediatric and adult cancer patients differ, as the former include late-stage complications and development/educational issues derived from a longer course of disease after treatment. Oral care, aiming to enhance patients’ QOL, is likely to be effective to address problems specific to pediatric cancer patients. This study examined the effects of specialized oral care to improve such patients’ oral environments and QOL.
Methods: Pediatric cancer in patients receiving chemotherapy and radiotherapy in our University Hospital Medical Center for Children, for whom specialized oral care had been requested, were studied. The contents of the specialized oral care for them included: brushing guidance, dental tartar removal, and oral moistening, gargling guidance, and/or dental treatment in accordance with the type of oncological treatment. Before and after care, oral environments were assessed using an original sheet, while measuring oral moisture and bacterial levels, and conducting a questionnaire survey on patient satisfaction. The assessment sheet consisted of Revised Oral Assessment Guide (ROAG) items, in addition to those regarding halitosis, the mouth-opening degree, dental condition, changes in the gustatory sense, and oral mucositis. For the questionnaire survey, the Child Perceptions Questionnaire (CPQ) 8-10 sheet was used to comprehensively evaluate the pediatric patients’ oral health-related QOL.
Results: The mean oral assessment score decreased after care, while there were no changes in the oral moisture or bacterial level. Regarding the questionnaire, the mean overall score decreased after care. Decreases were also observed in the score for each question with increases in the number of intervention sessions.
Conclusion: The maintenance and improvement of oral hygiene through specialized oral care are important to appropriately treat late-stage complications and provide health management education for pediatric cancer patients. In this study, specialized oral care was effective to improve such patients’ QOL.
oral care, pediatric cancer, perioperative period
Cancer is the leading cause of clinical death among children. Pediatric cancer is difficult to prevent than adult cancer because it is not associated with lifestyle-related diseases. In addition, the development of treatment and medicine for pediatric cancer is delayed due to the small number of cases. Pediatric cancer treatment at the growth and developmental stages is at risk for late-stage complications such as developmental disorders, endocrine disorders, organ disorders, gonadal disorders, brain dysfunction, the development of secondary cancers and the increase in economic and psychosocial burdens after treatment. Furthermore, issues such as returning to school, working, marriage and childbirth require long-term support for patient education and independence as well as for families. As mentioned above, QOL is impaired in pediatric cancer patients.
Oral care, aiming to enhance patients’ QOL by preventing and reducing adverse events or complications related to cancer treatment, is likely to be effective to address problems specific to pediatric cancer patients [1-3]. Therefore, the initiation of oral care-based intervention when a cancer diagnosis is established may also be useful in pediatrics. In pediatric cancer treatment, objective and subjective assessment indices differ, and they are under the marked influences of environmental factors. Studying adolescents’ and young adults’ QOL has been reported to be key to accurately predicting their health conditions as part of pediatric cancer treatment [4]. In order to provide high-quality oral care services, it is also important to promote active liaison between dentistry and medicine, and feed the results of subjective evaluation of the effects of dental approaches on pediatric patients’ QOL back to medical professionals [3].
Therefore, a study was conducted to clarify the effects of oral care on pediatric cancer patients by assessing their oral environments.
Subjects
Pediatric cancer in patients receiving chemotherapy and radiotherapy in the Medical Center for Children of the university hospital, for whom specialized oral care had been requested, were studied (Table 1).
Table 1. Details of cases
Case |
Age |
Sex |
Diagnosis |
Chemotherapy |
Duration of
hospital stays (Day) |
Radiation
(Gy) |
1 |
8 |
Male |
Leukaemia |
Cytarabine, methotrexate |
173 |
0 |
2 |
6 |
Male |
Leukaemia |
Methotrexate |
249 |
0 |
3 |
6 |
Female |
Neuroblastoma |
Carboplatin, etoposide |
644 |
100.8 |
4 |
9 |
Male |
Leukaemia |
Methotrexate |
221 |
50 |
5 |
12 |
Female |
Neuroblastoma |
Cyclophosphamide, dacarbazine |
3072 |
100.8 |
6 |
6 |
Male |
Leukaemia |
Mitoxantrone |
356 |
0 |
7 |
11 |
Male |
Medulloblastoma |
Carboplatin, etoposide |
667 |
55.8 |
8 |
6 |
Female |
Neuroblastoma |
Ifosfamide |
533 |
60.6 |
9 |
6 |
Female |
Osteosarcoma |
Pirarubicin |
486 |
0 |
Specialized oral care
The contents of the specialized oral care performed by dentists or dental hygienists for them included: brushing guidance, dental tartar removal, and oral moistening, gargling guidance, and/or dental treatment in accordance with the type of oncological treatment using behavioral modification (TSD method, operant conditioning with reinforcement, systematic desensitization method, 10 count method). Furthermore, in order to educate not only patients but also parents about the necessity of oral care, we visited frequently and gave lectures using media (picture-story show, model). Before and after the specialized oral care, oral environments were assessed using an original sheet (Table 2) based on the Revised Oral Assessment Guide (ROAG) developed by Andersson [5] and Common Toxicity Criteria for Adverse Events Ver. 3.0 (CTCAE) established by the National Cancer Institute (NCI) [6]. The sheet comprised questions regarding halitosis, the mouth-opening degree, dental condition, changes in the gustatory sense, and oral mucositis, in addition to the ROAG items. Subsequently, the oral moisture level in the central part of the dorsum of the tongue was measured using an oral moisture meter (Mucus®, LIFE, Co., Ltd., Saitama), while bacterial counting was performed using a bacterial counter (Bacterial Counter®, Panasonic Healthcare Co., Ltd., Tokyo).
Table 2. Oral assessment using an original sheet
ID |
|
|
Perioperative Cancer Assessment Sheet |
Note |
Name |
|
Sex |
1. Male
2. Female
|
|
Age
(yrs.)
|
|
1. 0-20 2. 21-30 3. 31-40
4. 41-50 5. 51-60 6. 61-70
7. 71- 80 8. 81-90 |
|
Anticancer drugs |
1. Yes 2. No |
|
Drug name (product name) |
|
Please indicate the name in the space to the right. |
|
Type |
Select the type from the following, and indicate it in the space to the right (multiple answers allowed) |
|
1. Alkylating drugs
2. Antimetabolic drugs
3. Plant alkaloids
4. Molecular-target drugs
5. Hormone drugs
6. Anticancer antibiotics
7. Biological response modifiers
8. Others |
Carcinoma |
Name of the diagnosis |
|
Please indicate the name in the space to the right. |
|
Classification |
1. Central/peripheral nervous system
2. Gastrointestinal tract (esophagus - rectum/anus), hepatobilipancreatic system
3. Hematopoietic/lymphoreticular system
4. Bone/soft tissue
5. Mammary gland, uterus, ovaries, fallopian tubes, placenta
6. Kidney, lower urinary tract (ureter, bladder, urethra), prostate, testicle
7. Lung, thymus, pleura
8. Pituitary gland, thyroid, parathyroid, adrenal gland
9. Entire craniocervical region
10. Skin |
|
Radiotherapy |
1. Yes 2. No |
|
|
Radiation site |
|
|
Radiation dose |
|
Radiation frequency |
|
Vocalization |
1: Normal |
2: Low or hoarse voice |
3: Difficult to speak |
|
Swallowing |
1: Normal swallowing |
2: Painful |
3: Unable to swallow |
|
Lips |
1: Smooth and pink |
2: Dry or cracked and/or angular cheilitis |
3: Ulcers or bleeding |
|
Tongue |
1: Pink and moist with the papillae visible
2: Dry with the papillae invisible or red/white
3: Very thick white coating, vesicles, or ulcers |
|
Saliva |
1: No resistance between the mirror and mucosa
2: Resistance slightly increases, but the mirror does not touch the mucosa
3: Resistance clearly increases, and the mirror touches or nearly touches the mucosa |
|
Mucosa |
1: Pink and moist
2: Dry and/or red, purple, or white
3: Marked redness or thick white coating |
|
Gingiva |
1: Pink and firm
2: Swollen and/or red
3: Easily bleeding when pressed by hand |
|
Tooth/denture cleanliness |
1: Clean
2: 1) Plaque and/or food residue in some parts; 2) Caries and/or denture damage
3: Plaque and/or food residue throughout the oral cavity d |
|
Halitosis |
1: Not perceivable
2: Perceivable at a distance of less than 30 cm from the mouth
3: Perceivable even at a distance of more than 30 cm from the mouth |
|
Mouth-opening degree |
1: Able to open the mouth independently without limitations
2: Able to open the mouth independently with limitations (a breadth of 2 horizontal fingers); Unable to open the mouth independently due to consciousness disturbance, but it is passively and manually openable
3: Mouth-opening is limited to a breadth of 1 horizontal finger or less due to bruxism or mandibular contracture |
|
Dental condition |
1: There is no tooth requiring dental treatment
2: There is a tooth/teeth that may interfere with care or be a source of infection
3: There is a tooth/teeth requiring early dental treatment such as tooth extraction and grinding |
|
Changes in the gustatory sense |
1: Unchanged
2: There are changes in the gustatory sense, but they do not influence diet
3: There are changes in the gustatory sense, influencing diet (e.g., oral intake of dietary supplements); an uncomfortable or unpleasant taste; a loss of taste sensation |
|
Oral mucositis |
1: Absent
2: Painless ulcer or erythema, or mild pain derived from unidentified lesions
3: Painful erythema, edema, or ulcer, but able to eat and swallow
4: Painful erythema, edema, or ulcer, and intravenous fluid injection is required
5: Severe ulcer, and tube or intravenous feeding or preventive intubation is required |
|
|
Site |
Lateral |
1. Left |
2. Right |
3. None |
|
|
Vertical |
1. Maxilla |
2. Mandible |
3. None |
|
Lesion |
Ulceration |
1. Painless |
2. Painful |
3. Tachetic |
4. Absent |
|
|
Erythema |
1. Present |
2. Absent |
|
|
Edema |
1. Present |
2. Absent |
|
|
Dysphagia/painful swallowing |
1. Present |
2. Absent |
|
|
Skin erosion |
1. Present |
2. Absent |
|
|
Pseudomembrane |
1. Present |
2. Absent |
|
|
Bleeding |
1. Bleeding due to mild external injury |
2. Tissue necrosis (major spontaneous bleeding) |
|
Graphical representation |
Please indicate any figures as a reference, if possible. |
Patient satisfaction questionnaire survey and totaling
A patient satisfaction questionnaire survey was conducted before and after the specialized oral care. The Child Perceptions Questionnaire (CPQ) 8-10 sheet (Table 3) was used to comprehensively evaluate the pediatric patients’ oral health related QOL [7]. Responses were scored as follows: 0: Never, 1: Once or twice, 2: Sometimes, 3: Often, 4: Almost every day/always.
Table 3. The child perceptions questionnaire (CPQ) 8-10 sheet

The study was approved by the medical ethics committee of our university (EMB-C-323).
Changes in oral environments
The mean ROAG score, as well as those related to halitosis, the mouth-opening degree, dental condition, changes in the gustatory sense, and oral mucositis, decreased after the specialized oral care (Figure 1).
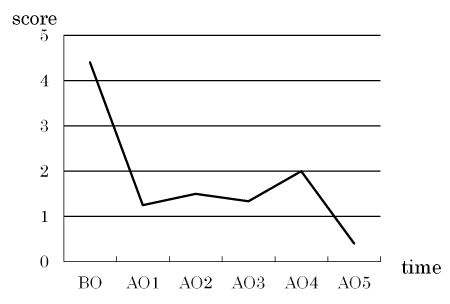
Figure 1. Changes in the mean assessment score
Oral moisture and bacterial levels
There were no intervention-related changes in the oral moisture or bacterial level (Figures 2 and 3).
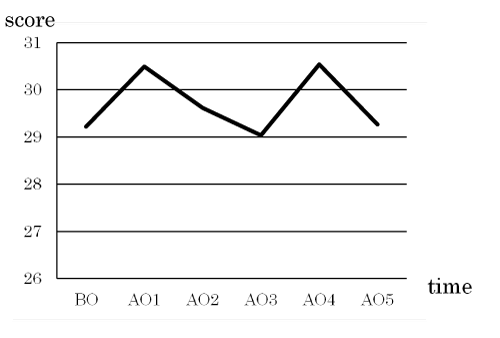
Figure 2. Changes in oral moisture
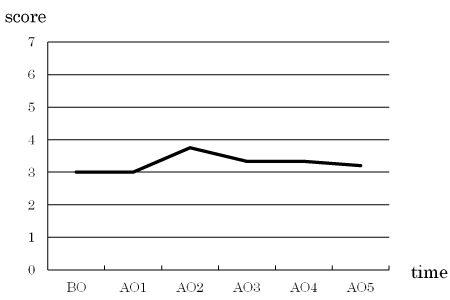
Figure 3. Change in bacterial count
Patient satisfaction questionnaire survey
The mean CPQ8-10 score, representing patient satisfaction, decreased after the specialized oral care (Figure 4). Decreases were also observed in the score for each question (regarding oral symptoms, functional limitations, or mental or social stability) with increases in the number of intervention sessions (Figure 5).
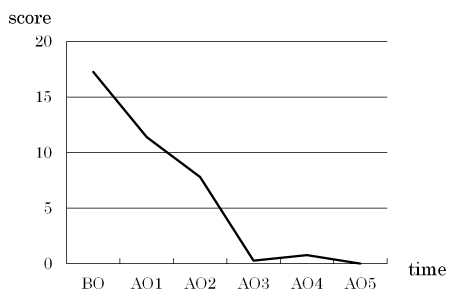
Figure 4. Changes in the mean CPQ8-10 score
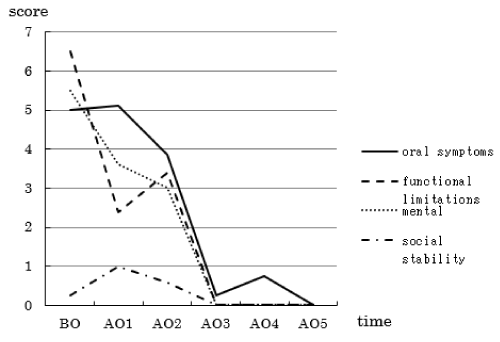
Figure 5. Changes in the mean score for each question
Time-dependent decreases in assessment scores were observed after the specialized oral care, indicating improved oral environments. On the other hand, there were no marked changes in the oral moisture or bacterial level, possibly due to the subjects’ young age and the consequent absence of a history of oral diseases. In fact, the oral moisture level was appropriate, and the bacterial level was 3 (1,000,000 cfu/mL) in the majority of patients before the intervention. As a result, specialized oral care may not have markedly influenced these items.
In the questionnaire survey, the CPQ score decreased after each intervention session, indicating an increase in the patient satisfaction level. After the fifth intervention session (Month 5), all of the scores related to oral symptoms, functional limitations, and mental/social stability decreased to 0, with the lowest assessment scores, confirming that the endpoints of specialized oral care had been achieved. In short, the oral hygiene status of patients with pediatric cancer requiring long-term inpatient treatment was improved or maintained at 5 months after the initiation of intervention. The results also highlighted the necessity of further promoting support, which is not unilaterally provided by medical/dental professionals, but also covers psychological considerations for children and their parents, when performing specialized oral care. In this respect, the study confirmed the feasibility of unilateral, subjective assessment, involving medical professionals and pediatric patients, in addition to quantifying the outcomes of specialized oral care. Considering that studies on the effects of such care or patient satisfaction with it have rarely been conducted to the present, the study may have been of marked significance by examining its effects on pediatric cancer.
As future perspectives, oral hygiene improvement and maintenance may be important to appropriately manage late-stage complications in pediatric cancer patients, while providing health management education for them, and these approaches are likely to contribute to such patients’ QOL.
On objectively evaluating specialized oral care for pediatric cancer patients, it was shown to improve their oral environments and satisfaction levels. Oral hygiene improvement and maintenance may be important to appropriately manage late-stage complications in pediatric cancer patients, while providing health management education for them, and these approaches are likely to contribute to QOL-oriented specialized oral care.
The progress of the research was advised and instructed by emeritus professor Yoshiyuki Watanabe. This research was conducted with research support for the 8020-research project.
- Chiyadu PK, Yellamma B (2014) Oral and dental considerations in pediatric leukemic patient. ISRN Hematology Artic
- 11. Stephan TS, Edward GF (2002) Oral complications of cancer therapy. Oncology 16: 680. [Crossref]
- da Fonseca MA (2004) Dental care of the pediatric cancer patient. Pediatr Dent 26: 53. [Crossref]
- Matsuda T, Noguchi M, Umeno Y, Kato N (2006) QOL research in child health present state and issues. Jpn J Public Health 53: 805. [Crossref]
- Pia A, Ingalill RH, Stefan R (2002) Inter-rater reliability of an oral assessment guide for elderly patients residing in a rehabilitation ward. Spec Care Dentist 22: 181. [Crossref]
- Common terminology criteria for adverse events v3.0 (CTCAE) National cancer institute (NCI): August 9, 2006. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf
- Yamada H, Miura H, Usui Y, Sato T (2013) Development of the Japanese version of the child perceptions questionnaire (CPQ8-10_JP). Pediatr Dental J 23: 86.






