Background: In Sri Lanka, the incidence of asthma has been reported as 2.6% in children. Increased cost and poor adherence of Inhaled Corticosteroids (ICSs) put forward the advancement of leukotriene antagonists like montelukast. However, the efficacy of montelukast has not been studied fully in children of Sri Lanka. The aim of this study is to compare the efficacy of montelukast and ICS in children aged 1 to 5 years with mild persistent asthma.
Methods: This randomized clinical trial was conducted from December 2011 - May 2013. Children age 1-5 years having cough and wheeze were included. The endpoint of the study was to determine the effectiveness of montelukast in the improvement of asthma symptoms measured by asthma score in comparison to ICS. Participants were divided into 2 groups where group 1 was designated for ICS and group 2 for montelukast. Data was recorded daily in the symptom diary, while coded and analyzed on SPSS. This trial is registered with Sri Lanka Clinical Trial Registry under number: SLCTR/2011/006.
Results: The study population of 81 children of which 64.2% were male, mean age was 40.12 ± 13.50 months and weight of 17.70 ± 21.65 Kg. Significant improvement in the mean asthma score was observed in both groups. As compared to ICS, nasal congestion was significantly reduced in montelukast group with mean score of 281.71 ± 27.05 (p = 0.034) and improvement in physical activities were equally achieved.
Conclusion: Montelukast is equally effective as inhaled corticosteroids in prevention of mild persistent asthma among 1-5 years and additional significant benefit in controlling rhinitis.
asthma symptoms, efficacy, inhaler corticosteroid, montelukast
Abbreviations
ICSs: Inhaled Corticosteroids; NAEPP: National Asthma Education and Prevention Program; FEV: Forced Expiratory Volume; SABA: Short acting β2 agonist; LABA: Long acting β2 agonist; ETU/ITU: Emergency/Intensive treatment Unit; SLMA: Sri Lanka Medical Association; URTI: Upper Respiratory Tract Infection; GINA: Global initiative for asthma; SIMPLE: SIngulair in Mild asthma: comPLiance and Effectiveness
Asthma is a common chronic obstructive pulmonary disorder that usually becomes the major reason of disability, economic burden and poor quality of life [1]. The global burden of Asthma has been reported as 4.3%, where the highest burden was observed in Western Pacific with 6.2%. South East Asia found to have 3.24% prevalence in which Sri Lanka has been estimated to have a 2.6% asthma burden [2]. It is evident through available data that risk of asthma is continuously prevailing and by 2025 the burden might reach to 400 million asthmatic patients globally. While children are known to be more susceptible in comparison to adults so, the risk is even higher [3,4].
According to National Asthma Education and Prevention Program (NAEPP) guidelines, the clinically indications of asthma include dyspnea, breathlessness, wheezing, cough and chest tightness [5,6]. In 2007-2009, NAEPP guidelines had classified asthma and its management into three severity grades where Intermittent asthma is characterized by presenting symptoms of wheezing, chest tightness not more than 2 times a week with 80% Forced Expiratory Volume (FEV). While all these symptoms in mild persistent asthma occur 3-6 times a week and nocturnal symptoms 3-4 times per month although FEV is 80%, moderate persistent asthma causes these symptoms to appear daily with a decreased FEV to 60% and severe asthma is characterized by these symptoms in a continual manner with FEV 60% [5,6]. Children are more prone to fall in intermittent or mild asthma category, which may be due to decreased effectiveness of the therapy, under-reporting of symptoms and increased chances of exposure to allergens in this age [7].
Complete relief from asthma is still not possible with existing therapeutic classes and this is assumed because of multiple etiological reasons. However, focusing on symptomatic treatment of pathophysiological features may help improving the quality of life in asthmatic patients. For example, conditions like bronchoconstriction and underline inflammation of airway comprises of major reasons of worsening of asthma [6]. Thus, the management pharmacotherapy is classified into β2 agonist, corticosteroids, cromolyn, methyl xanthine derivatives, antimuscarinic bronchodilators and leukotriene, on the basis of severity of the asthma [8]. NAEEP guidelines (2007-2009), follows management according to asthmatic classification i.e. for intermittent asthma short acting β2 agonist (SABA) are preferred. Mild persistent asthma can be treated with either low dose Inhaled Corticosteroid (ICS) or leukotriene while for moderate asthma low/medium dose ICS either with long acting β2 agonist (LABA) or with leukotriene. Severe asthma can be managed through medium dose ICS with LABA and leukotriene [5,6].
With the better understanding of other pathophysiological pathways of asthma, Leukotriene were found to be the important proinflammatory mediators in this condition [9]. Those release through a series of events and cause bronchoconstriction, mucus secretion and smooth muscle proliferation [8,9]. Antagonizing the effect of these mediators were not achieved by corticosteroids [10]. Hence, Montelukast, a novel leukotriene antagonist was designed and approved leukotriene antagonist for children with mild persistent asthma and since then a series of clinical trials have carried out to determine the efficacy and tolerability of this medicine in treating asthma [11-13]. ICS have always been a popular choice of treatment to manage asthma both in adults and children with utilization of both low and high dose ICS. Nevertheless, studies have also shown ICS are potentially harmful for children in long run, as it negatively effects long-term growth, bone mineralization and neuroendocrine functioning [11-13]. According to former studies, Montelukast can be prescribed as an alternative therapy of low-dose ICS since it was found to provide rather similar antagonizing effects like ICS on clinical indicators of asthma especially in intermittent or mild persistent asthma [11-14].
Moreover, keeping in consideration that studies on ICS have been carried out among its effect with placebo. Therefore, it will be handful to conduct a trial of ICS with Montelukast to provide ease for the physician in choosing the adequate therapeutic regime. Therefore, this randomized clinical trial is focused on evaluating the efficacy of oral Montelukast as compared to ICS in children of Srilanka with < 5 years of age in order to determine the long-term variation in efficacy of the study drug.
A randomized clinical trial was conducted from December 2011 to May 2013, at pediatric asthma clinic, faculty of medicine, University of Ruhuna, Sri Lanka .Children who were between the age group of 1-5 years and those who comes in the category of mild persistent asthma were selected- those who had asthma symptoms more than 2 days per week but not every day and night symptoms more than 3-4 times per month. A total of 81 children were enrolled in the study with each having a 100 days regime. The enrolled children were then divided into two groups according to a random number table. Group 1 was given the Beclomethasone dipropionate as ICS salt, 100 μg 1 puff twice daily and the other group i.e. Group 2 was prescribed with oral Montelukast (Montiget) 4 mg (standard dose of age group). Prior to enrollment into this study, a trained interviewer was asked to take a preliminary inquiry. Parents of selected children were explained with all the procedures and objectives of this study and were asked to fill an informed consent for the purpose of participation in trial. After which participants were enrolled as per inclusion and exclusion criteria. Children between the age of 1-5 years having a cough, wheezing as an asthmatic symptom were recruited while children with a history of respiratory distress, chronic lung disease other than asthma, pneumonia and Emergency/Intensive treatment Unit (ETU/ITU) hospital admission, premature birth of a child (less than 37 weeks ) and the use of long term medication (ICS or Montelukast) during last 3 months were excluded from the study sample.
All children were monitored fortnightly for improvements in asthmatic symptom and parents were educated to keep a record of any asthmatic distress in standardized daily asthma symptom diary. Information regarding economic, social and disease-related factors, control of symptoms, its severity and other contributing factors was collected from patients. The severity of asthma was assessed at the baseline and at the end of the study using the daily asthma symptom diary [15]. The symptom diary comprised of 4 parameters including, cough, wheezing, nasal congestion and physical activity with a severity score from 0-3 for each symptom, where 0 means the most severe condition and 3 associates with symptomatic relief on each day for 100 days (maximum score for 100 days was 300) [15]. Whereas control of asthma was also checked by keeping records of the medicine used, like β2 agonists, a short course of steroids, and nebulization
Ethical approval has been obtained from the Faculty of Medicine, University of Ruhuna and the clinical trial is registered in the registry of Sri Lanka Medical Association (SMA) under the registration number of SLCTR/2011/006 [16]. Efficacy of the montelukast (Montiget) was evaluated with regards of improving the asthma symptoms measured by asthma score in comparison to ICS at baseline and at follow-up visit. Data was coded and analyzed using SPSS V.22 Demographic details and patient’s history was presented with the help of descriptive statistics where all qualitative variables were represented by frequency and percentages while mean± S.D was used to present quantitative variables. Moreover, Independent sample-t-test was used to evaluate the effictiveness of the drugs in treating asthma symptoms during the study duration.
A total of 81 children participated with a mean age of 40.12 ± 13.50 months and having a mean weight of 17.70 ± 21.64 kg were enrolled, where 64.2% were male children (Table 1). Out of the total, 61 patients completed the study with 30 patients in Group 1 and 31 in Group 2 (Figure 1). In all patients, nocturnal symptoms occurred in the last week with a mean of 3.35 ± 2.59 days, while physical activity was restricted for 0.68 ± 1.55 days and duration of attacks were found to be 4.82 ± 3.60 times per year.
Table 1. Demographics and clinical characteristics of study participants
Variables |
(n = 80) |
Gender |
Male |
52 (64.2) |
Female |
29 (35.8) |
Weight (kilograms) |
17.70 ± 21.64 |
Age (months) |
40.12 ± 13.50 |
Age of onset (months) |
28.12 ± 14.64 |
Family history of asthma |
50 (61.7) |
Nebulizing history |
38 (46.9) |
Inhaled dose (times nebulized)/day |
0.91 ± 1.40 |
|
Housing conditions (child's home eminent) |
Roof |
Sheet |
42 (51.9) |
Tiles |
39 (48.1) |
Pol athu (Coconut leaf) |
0 |
Wall |
Cement |
76 (93.8) |
Clay |
3 (3.7) |
Wood |
1 (1.2) |
Floor |
Cement |
59 (72.8) |
Tiles |
22 (27.2) |
|
Precipitating Factors |
URTI |
70 (86.4) |
Cold Air |
60 (74.1) |
Night-time |
59 (72.8) |
Dust |
49 (60.5) |
Activities |
21 (25.9) |
Pets |
10 (12.3) |
Smoke |
13 (16) |
Others |
2 (2.5) |
|
Dietary restriction |
Ice cream |
59 (72.8) |
Yogurt |
47 (58.0) |
Milk rice |
30 (37.0) |
Pulses |
30 (37.0) |
Anamalu |
30 (37.0) |
Papaya |
16 (19.8) |
Watermelon |
15 (18.5) |
Mullin |
15 (18.5) |
Other |
5 (6.2) |
|
Bathing conditions |
Hot bath |
46 (56.8) |
Cold bath |
34 (42.0) |
*Values are given as Mean ± SD or n (%)
*URTI: Upper Respiratory Tract Infection |
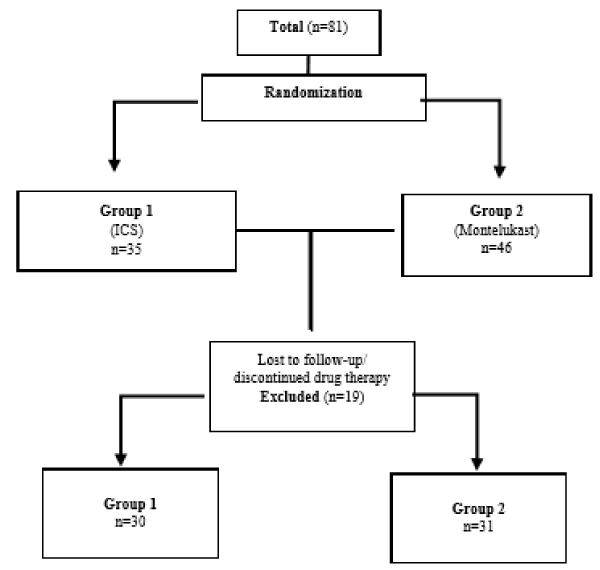
Figure 1. Flow of participants throughout the study period.
The common factors that triggers asthma were also observed, of them housing condition was focused as allergy to cement or sheets induces asthmatic attack. Other than that upper respiratory tract infections, cold air, nighttime, dust, pets and smoke were major reported precipitating factors. Bathing with hot water was considered to be a common factor by parents for asthma onset and therefore, it was observed that frequency of bathing among the patients also varied, i.e. 9 (11%) reported taking bath daily, 33(40.7%) opted bath for 2 to 6 times/week, 6(7.4%) patients used to take it fortnightly. While patients reporting monthly and once a year bathing pattern were 5(6.2%) and 3(3.7%) respectively. As for the dietary condition, ice cream was found to be the most restricted item among majority of the patients followed by yoghurt (Table 1).
Cough has been found to be the major problematic symptom of asthma followed by wheeze, runny nose and worsening symptoms after physical activity in participants of both the groups (Figure 2).
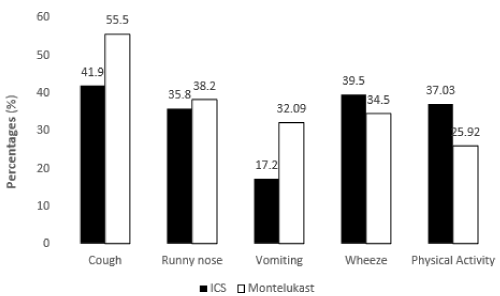
Figure 2. Symptoms before treatment among study participants.
Primarily both the agents were found to be most useful for treating asthma symptoms representing a similar trend in reduction. Nasal congestion was significantly reduced in group 2 (Montelukast) in comparison of group 1 (ICS) whereas, similar improvement was observed in daily physical activity among patients treated in both groups (Table. 2).
Table 2. Comparison of mean symptom among the treatment groups
|
|
Group 1 (ICS)
(n = 30) |
Group 2 (Montelukast)
(n = 31) |
p-value |
Symptoms (Total score) |
Cough |
282 ± 25.032 |
279.00 ± 24.928 |
0.641 |
Wheezing |
294.90 ± 7.83 |
287.55 ± 22.90 |
0.101 |
Nasal Congestion or Rhinitis |
265.13 ± 31.66 |
281.71 ± 27.05 |
0.032 |
Physical Activities |
293.51 ± 11.87 |
291 ± 16.41 |
0.899 |
Treatment |
|
|
|
Salbutamol inhalation |
37.40 ± 36.68 |
11.89 ± 33.07 |
0.102 |
*Values are given as Mean ± SD or n (%)
*ICS: Inhaled Corticosteroid |
The present study demonstrated that oral Montelukast and ICS both are effective against asthmatic symptoms in children under 5 years of age. However, Montelukast has been reported as a more satisfactory regimen in terms of soothing the symptoms like nasal congestion and shortness of breath induced by physical activity especially in mild persistent asthma. These findings are consistent with previous studies, indicating that Montelukast can be used as alternative of ICS [12-14].
Our results are in support of another study that states majority of patients receiving Montelukast were found to have rescue-free days in comparison to patients from ICS group [17]. Acording to a clinical study, shortness of breath induced by physical activity has been declined in patients when treated with Montelukast [18]. Comparable to our study results improved physical activities is supportive for the recommendations of Montelukast as a drug of choice for exercise-induced asthma, allergic rhinitis asthma and viral infection induced wheezing [12].
Many studies supported the efficacy of ICS in controlling severity of asthmatic symptom and hospitalization [19]. Hence, ICS are incorporated as first line treatment for asthma by Global initiative for asthma (GINA) [20]. As far as mild asthmatic treatment is concerned Montelukast is seemed to be a better choice as compared to ICS. Therefore, NAEPP & GINA guidelines recommend administration of Montelukast as an alternative of ICS and it could also be because of its equally effective profile, better adherence of the patient to the therapy, distinctive targeted pathway and economical availability of oral Montelukast [5,6,20,21,22]. It has been observed in Singulair in Mild asthma: compliance and Effectiveness (SIMPLE) trial, that Montelukast provided greater mild persistent asthma control as compared to ICS [23]. Since ICS substantially become an economic burden and because of its toxic side effects on the long-term growth of children, leukotriene can be used as long term therapy alternative to ICS [5,6,14]. Additionally, it has been proposed that leukotrienes (Montelukast) also play a vital role in repairing process of airway through cell proliferation and secretion mucosal fibroblast to remodel the damaged airway [22].
According to the previous literature Montelukast has effectively controlled asthma symptoms and also reduced Short-acting β-agonist (SABA) use (p < 0.001) [24]. And hence it was concluded that asthmatic symptoms were better controlled with Montelukast as compared to ICS, while a very few opposing responses were received [24]. Despite of the fact that ICS has been popular among asthma patients its use is now limited considering the side effects with increasing dose [25-27].
It is concluded that both therapeutic agents were effective in lowering the asthmatic symptoms of children between 1-5 years of age. However, nasal congestion was found to be significantly reduced in Montelukast than ICS whereas, daily physical activity showed similar reduction in both groups. Although, ICS are generally superior to Montelukast for asthma management but Montelukast also represents a safe and effective treatment option in children with mild persistent asthma and its use as an alternative to ICS has also been recommended globally.
We are thankful to Getz Pharma and Cipla pharmaceuticals in providing all required drugs for the study period.
We appreciate the support and the guidance given by Dr Thyagi Ponnamperuma- a Senior Lecturer in the department of Community Medicine, Faculty of Medicine, University of Ruhuna in the analysis of the data.
The authors are thankful to Ms. Mahwish Raza, Medical Affairs department of Getz Pharma for their assistance in the research and Advance Educational Institute & Research Center (AEIRC) & for their technical expertise in manuscript writing.
This is to declare that all authors have no significant competing financial, professional or personal interest that might have influenced the performance of data collection, manuscript writing or submission.
All 3 authors contributed in writing the proposal and data collection. For analysis the data first two authors contributed. Jayawardana PP and Imalke KACP.
Getz pharma and Cipla pharmaceuticals.
Montelukast was supplied by Getz pharma and metered dose inhalers (Asthalin and Beclate) were provided by Cipla pharmaceuticals.
- To T, Wang C, Guan J, McLimont S, Gershon AS, et al. (2010) What is the lifetime risk of physician-diagnosed asthma in Ontario, Canada? Am J Respir Crit Care Med 181: 337-343. [Crossref]
- To T, Stanojevic S, Moores G, Gershon AS, Bateman ED, et al. (2012) Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC public health 12: 204. [Crossref]
- Masoli M, Fabian D, Holt S, Beasley R (2004) Global Initiative for Asthma (GINA) Program: The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 59: 469-478. [Crossref]
- Akinbami LJ, Simon AE, Rossen LM (2016) Changing trends in asthma prevalence among children. Pediatrics 137: e20152354. [Crossref]
- National Asthma Education and Prevention Program (2007) Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin.Immunol 120: S94-138. [Crossref]
- Management of Asthma Working Group (2009) VA/DoD clinical practice guideline for management of asthma in children and adults. Washington (DC): Department of Veteran Affairs, Department of Defense; 2009.
- Bisgaard H, Zielen S, Garcia-Garcia ML, Johnston SL, Gilles L, et al. (2005) Montelukast reduces asthma exacerbations in 2-to 5-year-old children with intermittent asthma. Am J Respir Crit Care Med 171: 315-322. [Crossref]
- Jarvis B, Markham A (2000) Montelukast: a review of its therapeutic potential in persistent asthma. Drugs 59: 891-928. [Crossref]
- Negri J, Early SB, Steinke JW, Borish L (2008) Corticosteroids as inhibitors of cysteinyl leukotriene metabolic and signaling pathways. J Allergy Clin Immunol 121: 1232-1237. [Crossref]
- Caramori G, Adcock I (2003) Pharmacology of airway inflammation in asthma and COPD. Pulm Pharmacol Ther 16: 247-277. [Crossref]
- McIvor RA, Kaplan A, Koch C, Sampalis JS (2009) Montelukast as an aternative to low-dose inhaled corticosteroids in to management of mild asthma (the SIMPLE trial): An open-label effectiveness trial. Can Respir J 16: 11A-21A. [Crossref]
- Dubus JC, Marguet C, Deschildre A, Mely L, Le Roux P, et al. (2001) Local side‐effects of inhaled corticosteroids in asthmatic children: influence of drug, dose, age, and device. Allergy 56: 944-948. [Crossref]
- Kelly HW, Van Natta ML, Covar RA, Tonascia J, Green RP, et al. (2008) Effect of long-term corticosteroid use on bone mineral density in children: a prospective longitudinal assessment in the childhood Asthma Management Program (CAMP) study. Pediatrics 122: e53-61. [Crossref]
- Hon KL, Leung TF, Leung AK (2014) Clinical effectiveness and safety of Montelukast in asthma. What are the conclusions from clinical trials and meta-analyses? Drug Des Devel Ther 8: 839. [Crossref]
- Santanello NC, Davies G, Galant SP, Pedinoff A, Sveum R, et al. (1999) Validation of an asthma symptom diary for interventional studies. Arch Dis Child Fetal Neonatal Ed 80: 414-420. [Crossref]
- Srilanka Clinical Trial Registery (2011) Oral montelukast versus inhaled steroids for mild persistent asthma among 1 to 5 year old children. 2011 May 20.
- Phipatanakul W, Greene C, Downes SJ, Cronin B, Eller TJ, et al. (2003) Montelukast improves asthma control in asthmatic children maintained on inhaled corticosteroids. Ann Allergy Asthma Immunol 91: 49-54. [Crossref]
- Rubinstein I, Kumar B, Schriever C (2004) Long-term montelukast therapy in moderate to severe COPD—a preliminary observation. Respir Med 98: 134-138. [Crossref]
- Busse W, Raphael GD, Galant S, Kalberg C, Goode-Sellers S, et al. (2001) Low-dose fluticasone propionate compared with Montelukast for first-line treatment of persistent asthma: a randomized trial. J Allergy Clin Immunol 107: 461-468. [Crossref]
- Global Initiative for Asthma (2016) Global Strategy for Asthma Management and Prevention 2016. Global Initiative for Asthma (GINA).
- Maspero JF, Dueñas-Meza E, Volovitz B, Daza CP, Kosa L, et al. (2001) Oral montelukast versus inhaled beclomethasone in 6-to 11-year-old children with asthma: results of an open-label extension study evaluating long-term safety, satisfaction, and adherence with therapy. Curr Med Res Opin 17: 96-104. [Crossref]
- Paggiaro P, Bacci E (2011) Montelukast in asthma: a review of its efficacy and place in therapy. Ther Adv Chronic Dis 2: 47-58. [Crossref]
- McIvor RA, Kaplan A, Koch C, Sampalis JS (2009) Montelukast as an alternative to low-dose inhaled corticosteroids in the management of mild asthma (the SIMPLE trial): an open-label effectiveness trial. Can Respir J 16: 11A-16A.
- Reiss TF, Chervinsky P, Dockhorn RJ, Shingo S, Seidenberg B, et al. (1998) Montelukast, a once-daily leukotriene receptor antagonist, in the treatment of chronic asthma: a multicenter, randomized, double-blind trial. Montelukast Clinical Research Study Group. Arch Intern Med 158: 1213-1220. [Crossref]
- Garcia Garcia ML, Wahn U, Gilles L, Swern A, Tozzi C, et al. (2005) Montelukast, compared with fluticasone, for control of asthma among 6- to 14-year-old patients with mild asthma The MOSAIC study. Pediatrics 116: 360-390. [Crossref]
- Philip J (2014) The effects of inhaled corticosteroids on growth in children. Open Respir Med J 8: 66-73. [Crossref]
- Roland NJ, Bhalla RK, Earis J (2004) The local side effects of inhaled corticosteroids: current understanding and review of the literature. Chest 126: 213-219. [Crossref]


