Abstract
Aims: Identification of patients who benefit from mechanical circulatory support (MCS) after suffering an out-of-hospital cardiac arrest (OHCA) who have reached return of spontaneous circulation (ROSC) remains challenging. Increased phosphate levels have been reported as a negative prognostic marker for survival after OHCA. We aimed to investigate the impact of serum phosphate levels to identify patients who benefit from MCS implantation after OHCA with ROSC.
Methods: During 2016 and 2017 all adult patients admitted to our university hospital after OHCA, were included in our retrospective data analysis. As primary outcome, we compared survival to discharge grouped by first serum phosphate levels after arrival (cutoffs: ³2.5 and ³3 mmol/l) with and without following implantation of MCS.
Results: We included 95 Patients at a medium age of 64.7 ± 1.56 years. In 45 patients, serum phosphate exceeded ³2.5 mmol/l (32 ³3mmol/l). MCS was initiated in 22 patients, irrespective of initial serum phosphate. In patients w/o MCS, initial serum phosphate concentrations were higher in non-survivors compared to survivors (3 ± 0.17 vs. 1.8 ± 0.16 mmol/l, p< 0.001). However, in patients with MCS, elevated initial phosphate levels above 2.5 mmol/l were associated with greater chance of survival to discharge (cutoff 2.5 mmol/l: 39% vs. 9%, p=0.03; cutoff 3 mmol/l: 50% vs 9.1%, p=0.02). Phosphate clearance was higher in survivors and predicts MCS efficiency.
Conclusion: Elevated serum phosphate level after OHCA with ROSC may help to identify patients who benefit from MCS. Phosphate-clearance correlates with the effectiveness of MCS treatment to decrease individuals’ mortality.
Keywords
out-of-hospital cardiac arrest, MCS, phosphate, survival
Abbreviations
MCS: mechanical circulatory support; OHCA: out-of-hospital cardiac arrest; ROSC: Return of spontaneous circulation; (e)CPR: (extracorporeal) cardiopulmonary resuscitation; Dphosphate: phosphate clearance; SEM: standard error of the mean; AMI: acute myocardial infarction; ICU: intensive care unit
Introduction
Out of Hospital Cardiac Arrest (OHCA) remains a frequent cause of death in western countries, with an incidence of 1 per 1000[1]. Mechanical Circulatory Support (MCS) is increasingly used in OHCA patients especially as extracorporeal cardiopulmonary resuscitation (eCPR) with implementation under ongoing CPR despite metabolic derangements and long-lasting CPR and is mentioned as rescue therapy in the 2021 ERC Guidelines[2-4].
However, even after reaching a return of spontaneous circulation (ROSC), outcomes are only hard to predict and multiple treatment recommendations to optimize post-resuscitation care do exist[2]. In the last decades, several different parameters, such as time to ROSC, initial rhythm, bystander CPR or cause of cardiac arrest correlated with survival after OHCA [5,6] and therefore may help to interpret the patients’ chance for survival. Especially in patients with ongoing profound circulatory shock after reaching ROSC, strategies to improve hemodynamically stabilization and within this the possibility of myocardial and neurological recovery, are not well established. Besides complex medical treatment, some studies suggest the use of MCS devices to improve patients’ chance to recover after OHCA[7]. The identification of patients profiting from MCS remains a central and challenging task since MCS implementation is an invasive procedure accompanied by the individual risk of harmful side effects and high treatment costs despite limited resources in our health care systems. Established scores for risk stratification in patients with cardiogenic shock after OHCA were recently reported to show only moderate accuracy in patients treated with MCS [8]. Furthermore, parameters for prognostication of neurologic outcomes are not feasible at this early time point, since biomarkers such as NSE are only meaningful 48h after ROSC [9] and can be interfered by hemolysis [10,11] and the widely available brain imaging modalities are not able to predict good neurologic outcome so far[2].
Initial serum phosphate levels were shown to be associated with a poor chance of survival after out-of-hospital cardiac arrest[12,13], but so far, its value as a possible predictor for MCS implantation is not known. The impact of serum phosphate in this subgroup has not been investigated, although playing a crucial role in cellular energy production[14], maintaining the mitochondrial membrane potential[15], and contributing to the acidosis following cardiac arrest[16,17]. Therefore, here we aimed to analyze the impact of serum phosphate levels in patients following OHCA with ROSC stratified by implantation of MCS versus conservative shock management to evaluate if phosphate may be additionally used as a potential prognostic parameter for the identification of patients profiting from MCS.
Methods
Study design and population
This study was a retrospective observational single-center investigation, performed at Duesseldorf university hospital. 95 consecutive patients were included between 2016 and 2017. Patients were included after suffering an OHCA and reaching ROSC when they were aged ³18 years, had a non-traumatic reason for cardiac arrest and recorded serum phosphate at admission. Data were collected from the clinical data information system.
Our study has been approved by the local institutional ethics board (2018-153-KFogU) and due to its retrospective nature, no patient's consent was required.
As a primary outcome, we compared survival to discharge grouped by high or low first serum phosphate levels after arrival (cutoffs: ³2.5 and ³3 mmol/l) with and without implantation of MCS. Cutoffs were chosen within the range of increased risk for a bad outcome as described elsewhere[12]. Additionally, phosphate-clearance (DP) after MCS-Implantation was calculated in survivors and non-survivors.
ROC-Analyses based on initial serum phosphate in patients with and without MCS were performed.
CPR and mechanical circulatory support
Cardiopulmonary resuscitation was performed according to the 2015 guidelines published by the European Resuscitation Council[18]. MCS was used if indicated by the individual decision of the attending physician. Two different types of MCS were used: Impella CP (Abiomed, Danvers, MA) and Sorin Lifebox (Sorin Group, Munich, Germany). Subsequently, the patients were transferred to an intensive care unit, where target temperature management (TTM) with a target of 34° C was established for 24h, followed by 72 h normal body temperature.
Statistical analysis
Data are presented as means ± standard error of the mean (SEM). Statistical analyses were performed by using Fisher's exact test (case controls) and unpaired t-tests for detecting differences between the groups. Kaplan-Meyer analyses were performed using the Log-rank (Mantel-Cox) test. Significance was assumed if p was <0.05. Analyses were performed by GraphPad Prism version 9 for macOS.
Results
Included patients
Between 2016 and 2017 175 patients with OHCA were treated at the university hospital of Duesseldorf. Of those 67 did not reach ROSC, 8 received MCS before ROSC, and 5 patients had no recorded serum phosphate at admission and were excluded a priori (Figure 1).
We included 95 Patients at a mean age of 64.7 ± 1.56 years with a portion of 66.3 % males (Table 1). In 41.1 % an acute myocardial infarction (AMI), with or without acute ST-segment elevation, was deemed the cause of cardiac arrest by the interventionalist.
Table 1. Characteristics of patients suffering an OHCA after reaching ROSC dependent on MCS implementation and survival. MCS: mechanical circulatory support; w/o: without; ldh: lactate dehydrogenase; NSE: neuron specific enolase; AMI: acute myocardial infarction; CPR: cardiopulmonary resuscitation
Mean ± SEM, n=95 |
total |
MCS |
w/o MCS |
p-value |
population |
age [years] |
64.7 ± 1.56 |
56.3 ± 3.47 |
67.2 ± 1.64 |
0.003 |
male sex [%] |
66.3 |
63.64 |
67. 1 |
0.8 |
survival [%] |
36.8 |
36.4 |
37 |
1 |
initial laboratory values |
phosphate [mg/dl] |
2.6 ± 0.12 |
2.8 ± 0.20 |
2.5 ± 0.14 |
0.44 |
creatinine [mg/dl] |
1.7 ± 0.12 |
1.2 ± 0.09 |
1.8 ± 0.15 |
0.06 |
lactate [mmol/ l] (n=93) |
8.3 ± 0.62 |
7.3 ± 0.78 |
8.6 ± 0.78 |
0.4 |
potassium [mmol/ l] |
4.5 ± 0.12 |
4.1 ± 0.13 |
4.7 ± 0.14 |
0.03 |
ldh [U/l] |
748.1 ± 126.8 |
499.1 ± 48.07 |
823.1 ± 163.7 |
0.28 |
NSE [µg/l] (n= 87) |
122.2 ± 12.66 |
151.5 ± 27.42 |
112.2 ± 14.09 |
0.18 |
pH (n= 94) |
7.17 ± 0.03 |
7.21 ± 0.03 |
7.15 ± 0.04 |
0.44 |
CPR data |
AMI [%] |
41.1 |
59.09 |
35.62 |
0.08 |
bystander CPR [%] |
53.7 |
45.45 |
56.16 |
0.47 |
initial rhythm (VT/ VFib) [%] |
53.7 |
86.36 |
43.84 |
< 0.001 |
time to ROSC [min] |
31.3 ± 2.66 |
33.3 ± 4.43 |
30.7 ± 3.19 |
0.68 |
witnessed arrest [%] |
67.4 |
72.7 |
65.8 |
0.07 |
compression only CPR [%] |
49.5 |
40 |
60.3 |
< 0.001 |
|
total |
survivors |
non-survivors |
p-value |
population |
age [years] |
64.7 ± 1.56 |
60 ± 2.75 |
67.2 ± 1.82 |
0.03 |
male sex [%] |
66.3 |
70.8 % |
62.9 % |
0.5 |
initial laboratory values |
phosphate [mg/dl] |
2.6 ± 0.12 |
2.1 ± 0.18 |
2.9 ± 0.14 |
< 0.001 |
creatinine [mg/dl] |
1.7 ± 0.12 |
1.4 ± 0.1 |
1.8 ± 0.18 |
0.07 |
lactate [mmol/ l] (n=93) |
8.3 ± 0.62 |
5.5 ± 0.87 |
10.2 ± 0.78 |
< 0.001 |
potassium [mmol/ l] |
4.5 ± 0.12 |
4.4 ± 0.13 |
4.6 ± 0.17 |
0.32 |
ldh [U/l] |
748.1 ± 126.8 |
415.5 ± 31.42 |
942.1 ± 196.2 |
0.04 |
NSE [µg/l] (n= 87) |
122.2 ± 12.66 |
61 ± 7.79 |
156 ± 17.67 |
< 0.001 |
pH (n= 94) |
7.17 ± 0.03 |
7.26 ± 0.02 |
7.11 ± 0.04 |
0.01 |
CPR data |
AMI [%] |
41.1 |
57.14 |
31.67 |
0.02 |
bystander CPR [%] |
53.7 |
62.86 |
48.33 |
0.2 |
initial rhythm (VT/ VFib) [%] |
53.7 |
62.86 |
48.33 |
0.2 |
time to ROSC [min] |
31.3 ± 2.66 |
22.94 ± 2.872 |
40.13 ± 4.205 |
0.001 |
witnessed arrest [%] |
67.4 |
80 |
60 |
0.07 |
compression only CPR [%] |
49.5 |
40 |
55 |
0.2 |
Characteristics of CPR
Bystander CPR was performed in 53.7 % and the mean time to ROSC was 31.3 ± 2.66 min.
Shockable rhythm occurred in 53.8 % and was more often in patients who received MCS than in those who did not (86.4 vs. 43.8%, p< 0.001). A lower time to ROSC (22.9 ± 2.87 vs. 40.1 ± 4.21 min) and an assumed AMI (57.1 vs. 31.7 %) were associated with a greater chance of survival to discharge (Table 1). After witnessed cardiac arrest a trend towards a higher chance of survival occurred (80 vs. 60%, p= 0.071), whereas bystander CPR (62.9 vs. 48.3 %), initial shockable rhythm (62.9 vs. 48.3 %) and compression-only CPR (40 vs. 55 %) did not have statistically significant effects on survival.
Patients with and without MCS
Patients treated with and without MCS did not differ in sex, initial serum lactate (7.3 ± 0.78 vs. 8.6 ± 0.78 mmol/l), phosphate (2.8 ± 0.2 vs. 2.6 ± 0.1 mmol/l), NSE (151.5 ± 27.42 vs. 112.2 ± 14.09 µg/l), pH (7.2 ± 0.03 vs. 7.15 ± 0.04) or creatinine (1.2 ± 0.09 vs. 1.8 ± 0.15 mg/dl). No significant differences between the groups were observed in frequency of witnessed arrest (72.73 vs. 65.75%), bystander CPR (45.5 vs. 56.2 %), AMI (59.1 vs. 35.6 %) or time to ROSC (33.3 ± 4.32 vs. 30.7 ± 3.19 min).
Patients receiving MCS were younger (56.3 ± 3.47 vs. 67.2 ± 1.62 years), had more often a shockable rhythm (86.4 vs. 43.8 %), a lower incidence of compression-only CPR (13.64 vs. 60.27 %), and lower serum potassium levels at admission (4.1 ± 0.13 vs. 4.7 ± 0.14 mmol/l).
In patients with MCS, neither the time to ROSC nor an initial shockable rhythm, a witnessed arrest, performed bystander CPR, or compression-only CPR were associated with a higher chance of survival (Table 2). Full data are shown in tables 1 and 2.
Table 2. Characteristics of patients treated by MCS stratified by survival or non-survival. MCS: mechanical circulatory support; w/o: without; ldh: lactate dehydrogenase; NSE: neuron specific enolase; AMI: acute myocardial infarction; CPR: cardiopulmonary resuscitation
Mean ± SEM, n=22 |
total |
survivors |
non-survivors |
p-value |
CPR data |
|
|
|
|
AMI [%] |
59.1 |
50 |
57.1 |
1 |
bystander CPR [%] |
45.5 |
37.5 |
50 |
0.67 |
initial rhythm (VT/ VFib) [%] |
86.4 |
87.5 |
85.7 |
0.55 |
time to ROSC [min] |
33.3 ± 4.43 |
24.6 ± 4.16 |
30.4 ± 6.27 |
0.001 |
witnessed arrest [%] |
72.7 |
87.5 |
64.3 |
0.35 |
compression only CPR [%] |
13.6 |
12.5 |
14.3 |
1 |
Initial serum phosphate and lactate to predict mortality dependent on MCS implementation
Patients (with and w/o MCS) who survived until discharge had significantly lower initial serum phosphate (2 ± 0.18 vs. 2.9 ±0.14 mmol) and lactate (5.5 ± 0.87 vs. 10.2 ± 0.78 mmol/l) than those who died. Chance of survival (overall groups) did not differ in dependence of MCS (36.25 vs. 36.99 %).
In patients with initial serum phosphate ³ 2.5 mmol/l (n= 45), survival was found to be significantly higher with MCS implantation than without (39 vs. 9%, p= 0.03; Figure 2), even though there was no significant difference in serum lactate between the groups (9.4 ± 0.73 vs. 12.8 ± 1.2 mmol/l, p=0.08). The same observation applies for those with initial serum phosphate ³ 3 mmol/l (n= 32, 50 vs. 9.1 %, p=0.02). Kaplan Meyer analysis indicates a greater chance of survival in patients with ³2,5 mmol/l and ³ 3 mmol/l of initial serum phosphate and MCS than w/o MCS (Figure 3).
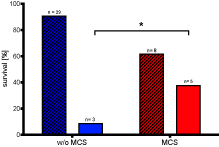
Figure 2. Patients with initial serum phosphate ≥ 2.5 mmol/l and MCS use had a greater chance to survive after OHCA following ROSC. Survivors to discharge (w/o pattern) and non-survivors (striped pattern). n=46 (w/o MCS: 33, MCS: 13), p= 0.02 for survival with and w/o MCS with Fishers exact test. MCS: mechanical circulatory support; w/o: without
In the time course following MCS-implantation, phosphate clearance was higher in survivors than in non-survivors (Figure 4B) when initial serum phosphate was ³ 2.5 mmol/l (2.9 vs. 1.7 mmol/l, p=0.02), whereas initial serum lactate was only predictive in patients without MCS (Figure 4D).
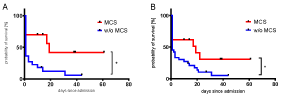
Figure 3. Kaplan-Meier-curve of patients with initial serum phosphate ≥ 3.0 mmol/l (A) or ≥ 2.5 mmol/l (B) and MCS (red) had a higher chance of survival than those without (blue). A: n=32, p< 0.01 with Log-rank (Mantel-cox) test. B: n=45, p= 0.03 with Log-rank (Mantel-cox) test. MCS: mechanical circulatory support; w/o: without
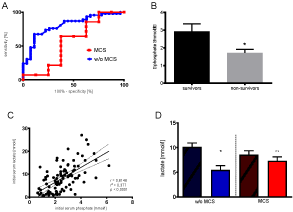
Figure 4. A: Initial serum phosphate predicts survival in patients who were treated without MCS (AUC 0.81, p< 0.001), but not in those treated with MCS (AUC MCS= 0.56 (p= 0.63)); with MCS n=2; w/o MCS n=73. B: phosphate clearance predicts the effectiveness of MCS use when initial serum phosphate was ≥2.5mmol/l; n=11, p=0.02 with Fishers exact test. C: Correlation between initial serum phosphate [mmol/l] and serum lactate [mmol/l] in OHCA patients after reaching ROSC and calculated regression curve with 95% confidence interval. r= 0.6146, r2=0.377, p< 0,001; D: Initial serum lactate predicts survival in patients reaching ROSC following OHCA treated w/o MCS (n= 71 p< 0.001), but not in patients treated with MCS (n= 22, p= 0.75 with unpaired t-test). Survivors to discharge are shown w/o pattern, non-survivors are shown with stripped pattern
Furthermore, in ROC-Analyses (Figure 4A) initial serum phosphate levels turned out as a good predictor of survival to discharge in patients w/o MCS (AUC= 0.81, p< 0.001) but failed to predict outcome in patients treated by MCS (AUC= 0.56, p= 0,63), highlighting phosphate clearance as parameter to detect effectiveness of MCS treatment. Initial serum levels of phosphate and lactate correlated strongly (r= 0.61, p< 0.001, Figure 4C).
Discussion
Main findings
Our main findings are:
Elevated initial serum phosphate after OHCA with ROSC is associated with a poor chance of overall survival.
- Patients with elevated initial serum phosphate after OHCA with ROSC may profit from MCS.
- High phosphate clearance after OHCA with ROSC is associated with an improved chance of survival when MCS was used.
- Initial serum phosphate levels after OHCA with ROSC were independent of chronic kidney disease.
To the best of our knowledge, we here demonstrate evidence for the first time, that initial serum phosphate level of patients after OHCA with ROSC may be used as an independent parameter to identify patients who benefit from MCS implantation to optimize an individual’s survival, independently of a possibly unknown chronic kidney disease. Furthermore, a good phosphate clearance highlights the effectiveness of MCS in those patients.
MCS devices are increasingly used in patients with ongoing cardiac arrest (eCPR) or predominant cardiogenic shock following AMI[3,19,20]. Despite a growing number of studies investigating this field, eligible criteria for patient selection and the effect of MCS treatment are lacking. Currently running randomized controlled trials (RCT) e.g., DanGer Shock (clinicaltrials.gov identifier: NCT01633502) or ECLS Shock (NCT03637205) will elucidate the effects of MCS in these groups. The first evidence of the impact of elevated phosphate levels after CPR to predict poor outcome was recently described, whereas those cohorts did not include patients in cardiogenic shock who are treated with MCS[12,13]. Since serum phosphate is commonly not available immediately as a point of care testing, the initial decision during ongoing cardiac arrest cannot depend on initial serum phosphate. However, in most patients with ongoing (cardiogenic) shock, the time to decide the optimal treatment option just begins when ROSC is achieved and the patient is admitted to an intensive care unit (ICU), where initial serum phosphate levels are known as part of clinical routine labor testing. Due to the suffered cardiac arrest and the following post-cardiac arrest syndrome[21], a complex and only rarely understood interaction of macro-and microcirculatory dysfunction, metabolic and coagulation disorders, strategies to improve patient's outcome are of particular interest. Clinical methods to evaluate alterations in patients’ hemodynamics are well-established (e.g. cardiac output monitoring by pulse contour analysis or a Swan-Ganz catheter), but they require complex technical equipment and may be associated with adverse events. The assessment of initial serum phosphate levels therefore can be used as an easily accessible method to screen patients, which may profit from further diagnostic to implement MCS.
To our very best knowledge, this is the first study comparing patients’ outcomes after OHCA with ROSC stratified by initial serum phosphate and MCS. Patients with initial serum phosphate ³ 2.5mmol/l (and ³ 3 mmol/l) after reaching ROSC in case of an OHCA had a greater chance of survival to discharge, provided they were treated with MCS implantation (Figure 2). This is supported by ROC-Analyses, since initial serum phosphate is a good predictor of survival in patients without MCS (AUC: 0.81), whereas initial serum phosphate in patients where MCS was implemented, failed to predict mortality (Figure 4A). This may be interpreted as an effect induced by MCS treatment, indicating a positive effect for overall mortality after the implementation of MCS devices. Furthermore, the evaluation of phosphate-clearance after MCS implantation can then be used as a parameter to evaluate the effectiveness of this treatment, whereas initial serum lactate failed to predict mortality after MCS implementation (Figure 4B and 4D).
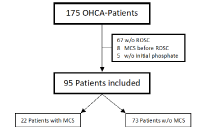
Figure 1. Flowchart representing the screened 175 patients with OHCA who reached the university hospital of Duesseldorf between 2016 and 2017. OHCA indicates out-of-hospital cardiac arrest; ROSC: return of spontaneous circulation; w/o: without; MCS: mechanical circulatory support
These findings may appear unexpected since high serum phosphate levels are known to correlate with poor outcomes[12,13], whereas one has to consider, that those studies did not include patients treated with MCS. However, our results are in line with current literature, since we also demonstrate a strong correlation of poor outcome and high initial serum phosphate levels in patients w/o MCS (Table 1) and do extend current knowledge: those patients with high serum phosphate levels and ongoing cardiogenic shock may profit from the implantation of MCS.
Under physiological conditions, the regulation of phosphate levels requires an intact hormone-gut-kidney axis to maintain phosphate homeostasis which is required for multiple cell-cell interactions e.g. in myocardial cells to regulate inotropic adaptation to circulatory changes[21-23]. Since the kidneys are responsible for phosphate-clearance and ongoing ischemia and hypoxia result in the release of intracellular phosphate, a relevant decrease in kidney perfusion, as present during CPR, increases serum phosphate levels, whereas its serum-amount correlates with the time of reduced organ and kidney perfusion. Furthermore, a return of spontaneous circulation is not mandatory associated with a regain of normal kidney perfusion, which therefore also reduces phosphate clearance and increases serum-phosphate levels. Therefore, the significant increase in serum phosphate levels cannot only be seen as an indicator of the previous ischemic period but its clearance can also be interpreted as an indicator of a poorly restored organ perfusion due to prolonged cardiogenic shock.
MCS implantation therefore may help those patients after CPR with primarily prolonged shock to improve organ perfusion, which improves the clearance of accumulated serum-phosphate. This allows a metabolic restart and production of high-energy phosphates to improve cardiomyocytes’ function, which is highly dependent on inorganic serum phosphate[14], and recover consequently. Our data support these hypotheses since survivors had a higher phosphate clearance than non-survivors (Figure 4B), which may be caused by regaining of kidney-phosphate-clearance and a regain of cellular phosphate consumption after initiation of MCS. Nevertheless, the underlying mechanisms of our findings are uncertain and need to be investigated in future studies.
Initial serum phosphate and lactate – where is the difference?
Among the studied population, a moderate correlation between initial serum phosphate and initial serum lactate was shown (Figure 4C). This is in line with the fact that both parameters correlate with the time of ischemia [12,24,25]. Serum lactate increases due to anaerobic glycolysis as a source of energy production, whereas phosphate levels increase in case of inadequate production of high energy phosphates, as a consequence of cell damage and reduced renal clearance. Good lactate clearance in patients treated with eCPR was recently reported to predict good neurological outcomes[26], which may be reasoned by adequate energy supply before irreversible brain damage occurred. Therefore, high and rising lactate levels do not only indicate ischemia but also indicate working anaerobic glycolysis with ongoing energy production. Increasing cell damage, as indicated by high serum phosphate, in patients with persisting shock after OHCA, can indicate the patient’s inability to restore energy production, which leads to death without MCS. This may explain the ability of serum phosphate to identify patients who need MCS and can explain the fact that survivors had a higher phosphate clearance than non-survivors (Figure 4B). Furthermore, our results indicate that single lactate evaluation is a worse predictive value for survival than phosphate, and therefore phosphate and its clearance can additionally be used as survival predictors when MCS is used.
High serum phosphate levels due to Chronic Kidney Disease?
Chronic kidney disease (CKD) may have influenced initial serum phosphate values and therefore biased our results[27]. However, creatinine did neither differ significantly between survivors and non-survivors (Table 1) nor did it between patients with and w/o MCS (Table 1), but it tended to be higher in non-survivors and patients w/o MCS. When calculating mean creatinine levels in patients with > 2.5 mmol/l of initial serum phosphate, differences between the groups become lower and the trend vanishes (date not shown). Furthermore, serum phosphate is only affected relevantly in patients with CKD stage 4 or 5[28,29]. Therefore, our main results are not reasonably explained by different stages of CKD in the studied population, and differences in creatinine levels between the groups may rather be affected by patients’ age, which was lower in survivors and recipients of MCS.
Limitations
Our study has several limitations, which have to been taken into account in data interpretation. At first its retrospective nature and a single-center report with a small number of patients without blinded investigators, which therefore forbid to conclude universality and causality. Second, the indication for or against MCS was made by the interventionalist on an individual basis and may have led to a selection bias. Furthermore, we did not analyze lactate clearance in comparison to phosphate clearance. Nevertheless, in our analysis, implementation of MCS was independent of serum lactate and patients with and w/o MCS did not differ in serum phosphate levels at admission (Table 1). Another limitation consists of the non-standardized time point of phosphate measurement in relation to OHCA.
Conclusion
Initial serum phosphate (³ 2.5 mmol/l) of patients after OHCA with ROSC can be used as an additional indicator to identify patients who benefit from MCS implantation to increase individuals’ chance of survival. In these patients, a higher phosphate clearance was associated with increased survival, which supports the effectiveness of MCS. Importantly, further prospective investigations are needed to confirm our results and clarify underlying mechanisms.
Acknowledgments
This work was performed in partial fulfillment of the requirements of an MD thesis of Laura Heyng.
Funding
This work was supported by the Forschungskommission of the Medical Faculty of the Heinrich-Heine-University Düsseldorf (No. 2018-50) and by the Christiane and Claudia Hempel Foundation, both to Ralf Erkens.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Smith TW, Cain ME (2006) Sudden cardiac death: epidemiologic and financial worldwide perspective. J Interv Card Electrophysiol 17:199-203. [Crossref]
- Soar J, Berg KM, Andersen LW, Bottiger BW, Cacciola S, et al. (2020) Adult Advanced Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation 156: A80-A119.
- Bartos JA, Grunau B, Carlson C, Duval S, Ripeckyj A, et al. (2020) Improved Survival With Extracorporeal Cardiopulmonary Resuscitation Despite Progressive Metabolic Derangement Associated With Prolonged Resuscitation. Circulation 141: 877-86. [Crossref]
- Ellouze O, Vuillet M, Perrot J, Grosjean S, Missaoui A, et al. (2018) Comparable Outcome of Out-of-Hospital Cardiac Arrest and In-Hospital Cardiac Arrest Treated With Extracorporeal Life Support. Artif Organs 42: 15-21.
- Martinell L, Nielsen N, Herlitz J, Karlsson T, Horn J, et al. (2017) Early predictors of poor outcome after out-of-hospital cardiac arrest. Crit care 21: 96. [Crossref]
- Hallstrom A, Herlitz J, Kajino K, Olasveengen TM (2009) Treatment of asystole and PEA. Resuscitation 80: 975-976.[Crossref]
- Goldsweig AM, Tak HJ, Alraies MC, Park J, Smith C, et al. (2020) Mechanical circulatory support following out-of-hospital cardiac arrest: Insights from the National Cardiogenic Shock Initiative. Cardiovasc Revasc Med P. S1553. [Crossref]
- Chatzis G, Markus B, Syntila S, Waechter C, Luesebrink U, et al. (2021) Comparison of Mortality Risk Models in Patients with Postcardiac Arrest Cardiogenic Shock and Percutaneous Mechanical Circulatory Support. J Interv Cardiol 2021: 8843935. [Crossref]
- Wihersaari L, Tiainen M, Skrifvars MB, Bendel S, Kaukonen KM, et al. (2019) Usefulness of neuron specific enolase in prognostication after cardiac arrest: Impact of age and time to ROSC. Resuscitation 139: 214-221. [Crossref]
- Geisen U, Benk C, Beyersdorf F, Klemm R, Trummer G, et al. (2015) Neuron-specific enolase correlates to laboratory markers of haemolysis in patients on long-term circulatory support. Eur J Cardiothorac Surg 48: 416-20. [Crossref]
- Mastroianni A, Panella R, Morelli D (2020) Invisible hemolysis in serum samples interferes in NSE measurement. Tumori pp: 79-81. [Crossref]
- Jung YH, Lee BK, Jeung KW, Youn CS, Lee DH, et al. (2018) Prognostic value of serum phosphate level in adult patients resuscitated from cardiac arrest. Resuscitation 128: 56-62. [Crossref]
- Lee HY, Jung YH, Jeung KW, Lee BK, Youn CS, et al. (2019) Ion shift index as a promising prognostic indicator in adult patients resuscitated from cardiac arrest. Resuscitation 137: 116-123. [Crossref]
- Pesta DH, Tsirigotis DN, Befroy DE, Caballero D, Jurczak MJ, et al. (2016) Hypophosphatemia promotes lower rates of muscle ATP synthesis. FASEB J 30: 3378-3387. [Crossref]
- Shibayama J, Taylor TG, Venable PW, Rhodes NL, Gil RB, et al. (2013) Metabolic determinants of electrical failure in ex-vivo canine model of cardiac arrest: evidence for the protective role of inorganic pyrophosphate. PLoS One 8: e57821. [Crossref]
- Makino J, Uchino S, Morimatsu H, Bellomo R (2005) A quantitative analysis of the acidosis of cardiac arrest: a prospective observational study. Crit care 9: R357-62. [Crossref]
- Oster JR, Alpert HC, Vaamonde CA (1984) Effect of acid-base status on plasma phosphorus response to lactate. Can J Physiol Pharmacol 62: 939-942. [Crossref]
- Monsieurs KG, Nolan JP, Bossaert LL, Greif R, Maconochie IK, et al. (2015) European Resuscitation Council Guidelines for Resuscitation 2015: Section 1. Executive summary. Resuscitation 95: 1-80. [Crossref]
- Bartos JA. (2020) The rise of the machines: ECLS and other temporary mechanical support for patients with cardiac arrest. Resuscitation 151: 208-210. [Crossref]
- Karatolios K, Chatzis G, Markus B, Luesebrink U, Ahrens H, et al. (2018) Impella support compared to medical treatment for post-cardiac arrest shock after out of hospital cardiac arrest. Resuscitation 126: 104-110. [Crossref]
- Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, et al. (2008) Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 118: 2452-2483. [Crossref]
- Onwochei MO (1993) Role of sodium/phosphate-cotransporter in myocardial contractile responses to inorganic phosphate. J Cardiovasc Pharmacol 22: 632-636. [Crossref]
- Marks J, Debnam ES, Unwin RJ (2010) Phosphate homeostasis and the renal-gastrointestinal axis. Am J Physiol Renal Physiol 299: F285-96. [Crossref]
- Smolenski RT, Swierczynski J, Narkiewicz M, Zydowo MM (1990) Purines, lactate and phosphate release from child and adult heart during cardioplegic arrest. Clin Chim Acta 192: 155-163.
- Dundar ZD, Cander B, Gul M, Karabulut KU, Kocak S, et al. (2012) Serum intestinal fatty acid binding protein and phosphate levels in the diagnosis of acute intestinal ischemia: an experimental study in rabbits. J Emerg Med 42: 741-747. [Crossref]
- Jung C, Bueter S, Wernly B, Masyuk M, Saeed D, et al. (2019) Lactate Clearance Predicts Good Neurological Outcomes in Cardiac Arrest Patients Treated with Extracorporeal Cardiopulmonary Resuscitation. J Clin Med p. 8. [Crossref]
- Malberti F (2013) Hyperphosphataemia: treatment options. Drugs 73: 673-688. [Crossref]
- Craver L, Marco MP, Martinez I, Rue M, Borras M, et al. (2007) Mineral metabolism parameters throughout chronic kidney disease stages 1-5--achievement of K/DOQI target ranges. Nephrol Dial Transplant 22: 1171-1176. [Crossref]
- Slatopolsky E, Robson AM, Elkan I, Bricker NS (1968) Control of phosphate excretion in uremic man. J Clin Invest 47: 1865-1874. [Crossref]




