Abstract
Maraviroc, originally designated UK-427857, is a small molecule and the first oral antiretroviral drug in the CCR5 receptor antagonist class used in the treatment of HIV infection. This drug classed as an entry inhibitor, was developed by the drug company Pfizer in its UK labs located in Sandwich. On April 24, 2007 the U.S. Food and Drug Administration advisory panel reviewing maraviroc's New Drug Application unanimously recommended approval for the new drug, and the drug received full FDA approval on August 6, 2007 for use in treatment experienced patients. Maraviroc is extensively metabolized by CYP3A4, with renal clearance accounting for approximately 23% of total clearance and has been shown to achieve an undetectable HIV-1 RNA level in clinically advanced, class three antiretroviral treatment-experienced adults with evidence of CCR5-tropic HIV-1 replication despite ongoing antiretroviral therapy. It is well tolerated, and its development is responding to a desperate need for new classes of antiretroviral agents that can target novel steps of the HIV lifecycle and do not share cross resistance with currently available therapy. This CCR5 receptor antagonist reviews clinically relevant pharmacological, long term therapeutic efficacy.
Our case report aims to explain the impact of Maraviroc co-administered with agents from all classes of antiretroviral therapy in a HIV-1 experienced patient along eleven years of antiretroviral experience.
Key words
HIV-1 infection, long-term maraviroc observation, limited side effect
Background
Maraviroc, is a small molecule and the first oral antiretroviral drug in the CCR5 receptor antagonist class used in the treatment of HIV infection and it has proven potent efficacy in treatment-experienced patients with multiple drug failure.
This drug classed as an entry inhibitor, was developed by the drug company Pfizer in its UK labs located in Sandwich.
On April 24, 2007 the U.S. Food and Drug Administration advisory panel reviewing maraviroc's New Drug Application unanimously recommended approval for the new drug, and the drug received full FDA approval on August 6, 2007 for use in treatment experienced patients [1]. Maraviroc is extensively metabolized by CYP3A4, with renal clearance accounting for approximately 23% of total clearance and has been shown to achieve an undetectable HIV-1 RNA level in clinically advanced, class three antiretroviral treatment-experienced adults with evidence of CCR5-tropic HIV-1 replication despite ongoing antiretroviral therapy. It is well tolerated, and its development is responding to a desperate need for new classes of antiretroviral agents that can target novel steps of the HIV lifecycle and do not share cross-resistance with currently available therapy [2]. This CCR5 receptor antagonist reviews clinically relevant pharmacological, long term therapeutic efficacy. Our case report aims to explain the impact of Maraviroc co-administered with agents from all classes of antiretroviral therapy in a HIV-1 experienced patient along eleven years of antiretroviral experience.
Case description
Our patient, female with HIV-1 infection diagnosed in September 1989, heterosexual 46-year-old Italian, presenting in the Hospital of Ancona (January 2008) without coinfections and at baseline CD4 count of 116 cells/μl, HIV-RNA 2604 cp/ml (detection limit 50 copies/ml). In January 2008, she started Maraviroc, zidovudine, lamivudine, darunavir, ritonavir, attended clinic regularly and reported good treatment adherence.
Her multidrug-resistance profile at baseline is (test TRUGENE HIV-1): reverse transcriptase mutations M41L, K65R, K70R, V75I, F77L, Q151M; protease inhibitor mutations L10V, K20R, L33F, M36I, M46I, G48V, I54T, L63P, A71V, V77I, V82I, I84V.
At time of admission in our Department of Infectious Diseases laboratory analysis are: AST 19 IU/ml (reference range, 1-36 IU/l), ALT 23 IU/ml (reference range, 1-36 IU/l), total bilirubin 0,4 mg/dl , triglycerides 152 mg/dl, total cholesterol 269 mg/dl, HDL cholesterol 59 mg/dl, creatinine 0,70 mg/dl., glycemia 82 mg/dl without history of insulin resistance., hypocomplementemia, glomerulonephritis, and autoimmune disorders and lipodystrophy. After two months of therapy the patient reveals (at objective examination) a mixed lipodystrophy. In April 2008 she switched to AZT/3TC, which was modified to tenofovir and emtricitabine for drugs intolerance. During subsequent follow up our patient has been maintaining good clinical conditions and a discrete adherence to HAART regimen and limit side effects.
In May 2010 biochemical , hematological and viro immunological parameters have demonstrated a good response to Maraviroc without intolerance, drugs resistance and side effects: CD4 T-cell count of 442 cell/mm3 and undetectable plasma HIV RNA concentration (detection limit 50 copies/ml) , total bilirubin 0,50 mg/dl , triglycerides 153 mg/dl, total cholesterol 271 mg/dl, HDL cholesterol 52 mg/dl, creatinine 0,80 mg., glycemia 73 mg/dl ALT concentration to 13 IU/l and AST 20 IU/l (reference range, 1-36 IU/l) and objective signs of lipodystrophy have decreased.
In July 2017 our patient switched PRESISTA (Darunavir/ritonavir) to REZOSTA (Darunavir 800mg+Cobicistat 150mg) for antiviral simplification.
In July 2018 she switchet TRUVADA (TDF+FTC) to DISCOVY (TENOFOVIR ALAFENAMIDE/EMTRICITABILE) for low compliance. Currently, after eleven years of therapy with Maraviroc, tenofovir, emtricitabine, ritonavir, darunavir, cobicistat laboratory examination and objective examination reveals a very important reduction signs of mixed lipodystrophy without any interruption of antiretroviral therapy containing protease inhibitors as backbone. (Figures 1 and 2)
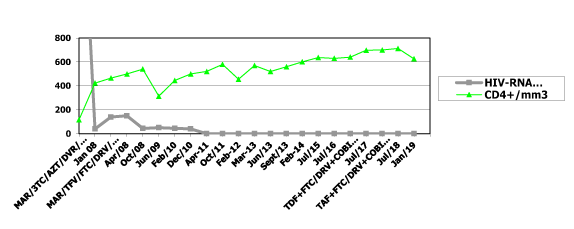
Note: MAR: maraviroc; COBI: cobicistat; TAF: tenofovir alafenamide; AZT: zidovudine; TFV: tenofovir; FTC: emtricitabina; DAV: darunavir; RTV: ritonavir; COBI: cobicistad; 3TC: lamivudine; DRV: darunavir
Figure 1. HIV-RNA viral load, CD4+ cell count and antiretroviral treatment
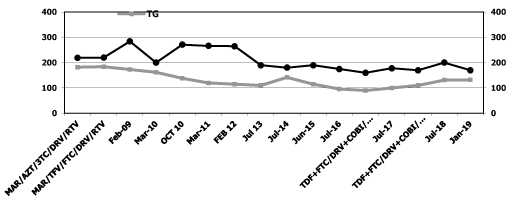
Note: MAR: maraviroc; COBI: cobicistat; TAF: tenofovir alafenamide; AZT: zidovudine; TFV: tenofovir; FTC: emtricitabina; DAV: darunavir; RTV: ritonavir; COBI: cobicistad; 3TC: lamivudine
Figure 2. Serum transaminases levels
In January 2019: CD4 T-cell count of 626 cell/mm3 and undetectable plasma HIV RNA concentration (detection limit 50 copies/ml), AST 17 IU/ml, ALT 19 IU/ml, total bilirubin 0,5 mg/dl, triglycerides 132 mg/dl, total cholesterol 200 mg/dl, HDL cholesterol 72 mg/dl, creatinine 0,85 mg., glycemia 81 mg/dl.
Surgery (excision or liposuction) has not been performed on our patient because his severe fat accumulation was reduced without the interruption of protease inhibitors.
This case demonstrates that MARAVIROC in combination with DARUNAVIR/COBICISTAT (REZOLSTA 800 mg/150 mg film-coated tablets) and TENOFOVIR ALAFENAMIDE /EMTRICITABINE (DESCOVY 200 mg/10mg) was effective in suppressing the viral load of a highly treatment experienced patient with HIV-1.
In treatment experien2021 Copyright OAT. All rights reserv with HIV-1 infection, MARAVIROC has been shown to have maximum benefit when introduced with at least one other active new antiviral agent and has been observed a limit side effect.
Discussion
Eradication of HIV infection cannot be archived with existing regimens. The goals of therapy are the prolonged suppression of viral levels to less than detection limits (<50 copies/mL for Amplicor assay, <75 copies/ mL for VERSANT assay, and <80 copies/mL for Nuclei Sens assay), with the aim to restore and preserve immunologic function, improve quality of life, and avoid HIV-associated morbidity and mortality. Treatment success needs strict lifelong drug adherence and Maraviroc monotherapy has a high potency and long half-life, allowing single-pill dosing.
Maraviroc is only effective against CCR5-tropic virus, which predominates throughout infection but is more common in patients at the early asymptomatic stage of infection. It is not known how quickly resistance may develop to maraviroc in clinical practice. Current evidence supports the continued development of maraviroc as a potentially useful, alternative treatment for the management of HIV infection
Our patient, experienced and multidrug resistant, has never stopped Maraviroc along six years of antiretroviral therapy and therefore has not developed any resistance.
Preliminary evidence indicates that maraviroc is likely to provide an alternative therapy for treatment-experienced patients, and for treatment-naïve patients who are newly infected with drug-resistant virus. However, improvements in efficacy or short- and long-term side effects for maraviroc compared with currently available regimens in treatment-naïve patients could positively impact on it use in this patient population provided that its use does not promote the selection of X4 HIV and more rapid disease progression. Approximately 50–60% of treatment-experienced patients and80–85% of treatment-naïve patients are infected with the CCR5-tropic virus only. A viral tropism test (Monogram Biosciences, San Francisco, CA, USA) is available to determine the probability of successful treatment, but the cost and turnaround time (3-5 weeks)
In summary, long term maraviroc therapy, meets an unmet need for a well-tolerated drug that reduces viral load with limit side adverse events in a HIV-1 experienced patient with preexisting class resistance.
Current evidence supports the continued development of maraviroc as a potentially useful, alternative treatment for the management of HIV infection. Its favorable toxicity profile makes the drug attractive for consideration in other clinical situations, including patients with earlier stages of disease, cardiovascular risk and viral hepatitis coinfection.
Conflicts of interest
The authors do not have a commercial or other association that might pose a conflict of interest.
Financial support
None
References
- Krauskopf L (2007) Pfizer wins U.S. approval for new HIV drug. Reuters
- Shilpa S, Homayoon K (2009) Maraviroc: a new CCR5 antagonist. Expert Review of Anti- infective Therapy 7: 9-19.


