Background
There are conflicting data about the relationship between diabetes mellitus and cancer risk in that growing evidence suggest a possible role of endogenous elevated insulin level which is often found in Non-Insulin-Dependent-Diabetes-Mellitus or an exogenous hyperinsulinemia observed often in Insulin-Dependent-Diabetes-Mellitus. In the last years higher attention focused on the role of immunoregulation both in the insulin production by β-cell of Langerhans-islets and in the insulin sensitivity (resistance) of insulin receptors. Interestingly, cytokines from type-2 innate immune cells, such as M2 macrophages or D2 dendritic cells exhibit a protective effect on both types of diabetes. However, the effect of insulin on the balance between type-1 and type-2 natural immune mechanisms, which is important for the tumor defense, was poorly investigated.
Material and methods
Streptozotocin (STX)-treated Wister rats was treated per oral with a non-optimal single dose of an evidence based and standardized plant immunomodulator, namely Rice Bran Arabinoxylan Concentrate (RBAC) which has been shown to activate type-1 innate immune cells (such as M1, D1 and NK cells). 24h after a single dose of RBAC (45mg/kg) four parameters of NK cells were determined with flow cytometry using stained CD161-APC and CD314-PE monoclonal antibodies and by hematological examinations. The results were compared with negative controls (without STX or RBAC treatment).
Results
Since STX caused a significantly reduced lymphocyte production in bone marrow, only the RBAC-induced relative increases in number of NKR-P1+ and LGL cells among the all lymphocyte population parallel with the frequency and intensity of the most important Killing Activator Receptor, namely NKG2D among the total NK cell population were determined. In the STX-untreated group RBAC induced only not significant increases compared with negative control values. However, in STX-treated groups all four NK parameters revealed RBAC-induced significant increases (p<0.05) compared to the negative controls.
Conclusion
These results suggest the hypothesis that insulin deficit can increase the immunomodulator-induced activation of type-1 innate immune cells indicating that insulin takes part on regulation of the natural immune balance inhibiting the type-1 innate antitumor defense.
insulin, NK-cells, NKG2D, Streptozotocin, LGL, Rice Bran Arabinoxylan Concentrate (RBAC), immunomodulation
The mechanistic process that may link diabetes mellitus to cancer is not completely understood yet. A great number of studies support a relationship between diabetes and increased risk of cancer in that endogenous or exogenous increased insulin level appears to play an important role [1-6].
The investigations to find an association between insulin and cancer risk are mostly carried out at patients with type-2 Non-Insulin-Dependent-Diabetes-Mellitus (NIDDM). The patients with type-1 Insulin-Dependent-Diabetes-Mellitus (IDDM) with progressive destroyed beta cells have often a non-physiological insulin replacement which does not match insulin needs and done to avoid hyperglycemia and its consequences [7,8]. As it well known, patients with NIDDM have an insulin resistance in spite of normal or over-production of insulin by the beta cells with often not reduced islet mass. In addition, no abnormalities are apparent either in the structure of insulin receptor or in the for signalization-responsible tyrosine-kinase. In vivo studies have it also demonstrated that in NIDDM the number of insulin receptors show no or only a very modest decrease. However, the insulin-mediated stimulation of tyrosine-kinase and autophosphorylation is impaired resulting in the insulin resistance (i.e. decreased sensitivity to insulin action by the receptors). It is also clear that the major locus of insulin resistance is distal to the receptor for which a very complex regulation is responsible. This lower responsiveness of insulin-receptors can explain the increased insulin level observed often in patients with NIDDM which are often discussed to play a role in a possible increased cancer risk. For example, in a meta-analysis with a great number of diabetes patients a comparison between insulin-use-group with non-insulin-group revealed a statistically significant association between insulin therapy and cancer [9]. The question, as to whether insulin can decrease the tumor defense mechanisms, was until now poorly investigated.
Since insulin and its precursors have been shown to have some homology to the Insulin-like Growth Factors (IGFs), an association between insulin and carcinogenesis appears to be plausible. Indeed, IGF-1 receptors, which can bind IGF-1 and IGF-2 with high affinity, are also able to bind Insulin with 100-fold less affinity resulting in only a very weak mitogenic effect [10]. Since the relationship between cancer-risk and insulin is not yet understood arises the question about the impact of insulin on immunoregulation. This is becoming increasingly relevant since in the last decade a polarization of innate immune system was discovered which appears to be strongly associated with the polarity of neuroendocrine system. Namely, the innate immune system is committed in two directions.
As shown in Figure 1, the type-1 macrophages (M1) and from monocytes originated type-1 dendritic cells (D1) generate proinflammatory cytokines such as interleukin (IL)-1 (only at short time), facilitate the production of IL-12, activate cytotoxic effectors, such as natural killer (NK), gamma-delta T and type-1 NKT1 cells which are potent inhibitors of tumor growth in MHC unrestricted manner [11-13]. However, these type-1 innate immune cells are down regulated in tumor disease [13]. Moreover, available information suggests that there is a tumor-induced dominance of type-2 macrophages (M2) and of the from the plasmocytoid precursors originated type-2 dendritic (D2) cells which generate IL-4 and IL-10 facilitating the generation of Th2 cells and inhibiting the type-1 system. It was also shown that M2 and D2 cells affect chronic inflammation, promote cell proliferation by producing growth factors and stimulate the angiogenesis. Parallel with the down regulation of type-1 cells, it was also found that tumor patients can have up to 40% type-2 peripheral monocytes in contrast to healthy persons who have only 10% [11,12]. In the tumor immunological research there are always more data which support this polarity. For example, in the patients with pancreas cancer, which has mostly a very unfavorable prognosis, the dominance of type-2 mechanisms appear to play a considerable role. It has been repeatedly shown that the peripheral level and cytotoxic activity of NK cells are positively correlated with their survival [14,15]. New evidences indicate that pancreas carcinoma cells release metabolites favoring the expansion and accumulation of monocytic-myeloid derived suppressor cells resulting in an increased level of both circulating and tumor infiltrating type-2 innate immune cells and this disturbed balance is associated with its shorter overall survival [16].
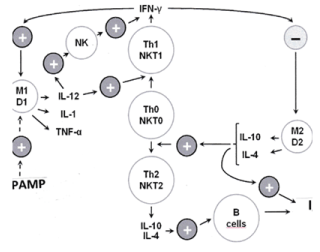
Figure 1. Innate immune system omitted in two directions. M1 and D1 are type-1 macrophages and dendritic cells which take part on the regulation of anti-tumor natural killer (NK and NKT1) cells serving as a relatively rapid defense. NK cells together with Th1 cells play a key role in the regulation of type-1 immune system. The alternative type-2 phagocytic cells (M2 and D2), when exposed to Th2 cytokines such as IL-4 and IL-10, represent a regulatory phenotype producing cytokines, such as IL-10 and Transforming Growth Factor (TGF)-beta which inhibit the type-1 system
A relationship between the immunoregulation and insulin sensitivity is also established [17-19]. As it will be discussed later the type-1 innate system appears to decrease and typ-2 system seems to increase the sensitivity of insulin receptors. Therefore, the present study is connected with surprising observations of previous experiment in rats [20] in that a microinjection of Interleukin (IL)-1β into the nucleus accumbens, which belongs to the limbic structures in the medial mediodorsal prefrontal cortex, resulted in an elevated glucose level established by the acute glucose tolerance test (GTT). Since IL-1β plays a key role in the activation of type-1 innate immune cascade mechanism (see Figure 1), present study directed to investigate the association between insulin and type-1 natural immune defense. Wistar rats were treated with Streptozotocin (STZ) which is known to selectively destroy β-cells of the Langerhans-islet inducing a type-1 diabetes. After establishment of destroying effect of STZ on β-cells using GTT, the effect of an evidence based and standardized plant immunomodulator, namely Rice Bran Arabinoxylan Concentrate (RBAC) on type-1 natural immunity was investigated. For this reason, the relative increases in peripheral NK cell frequency and their activating receptors were determined with and without previous STZ treatment. Our results support an inhibitory effect of insulin on the NK responses indicating a possible role of insulin in the regulation of natural immune defense which in the insulin-induced increase of cancer risk can also play an important role.
Animals
Thirty adult, male Wistar rats weighing 300-380 g were used in our present series of experiment. Animals were housed individually in a room with a 12-12h light-dark cycle. Constant room temperature (21 +/-2°C) and humidity (60+/-5%) were assured. Tap water and ordinary laboratory chow were ad libitum available. Rats were kept and cared for in accordance with institutional, national and international regulations with a permission of Animal Welfare Committee University Pécs (Nr.: BAI/35/616-5/2018).
Streptozotocin (STZ)
STZ was kindly provided by Department of Pharmaceutical Chemistry in University Pécs. As it well known, STZ can selectively destroy beta cells of the Langerhans-islet, so it is widely used to induce experimental typ-1 diabetes mellitus in animals [21-23]. Nine days before the blood test, 65mg/kg STZ was injected into the tail vein. After a week the animals were investigated with glucose tolerance test in order to exclude the non-diabetic animals.
Flow cytometry
Ten days after intravenous STZ administration, the animals were killed according to a government decree (40/2013/II.14 – in Annex 4) that followed 2-3 ml of blood to be down. For Flow Cytometric assay approximately 100 µl of blood in EDTA tubes was diluted with PBS containing 1% BSA, 0.02M EDTA, 0.02% sodium azide (PBS-BEA) and centrifuged at room temperature. The cells were resuspended in PBS-BEA and stained with appropriate monoclonal antibodies (mAbs) according to the standard staining procedure. The mAbs for flow cytometry were purchased from Merck KGaA (Darmstadt). For CD3 determination CD3-FITC clone, for NKR-P1 CD161-APC clone and for NKG2D examination CD314-PE clone were used.
Hematological investigation
Total leukocyte numbers were counted and from Giemsa-stained blood smears the differential leukocyte counts and LGL frequency among lymphocytes were determined by morphological examinations.
Rice bran arabinoxylan concentrate (RBAC)
RBAC belongs to the most investigated and most evidence-based plant immunomudulator. It is an arabinoxylan concentrate from rice bran (RBAC) which is manufactured and supplied in a standardized form as BioBran/MGN-3 by Daiwa Pharmaceutical Co, Ltd, Tokyo, Japan. It is composed of denaturated hemicellulose, which is obtained by rice bran hemicellulose reacting with multiple carbohydrate hydrolyzing enzyme from shiitake mushrooms. RBAC preparations are standardized for its main chemical component: arabinoxylan with a xylose (in its main chain) and with arabinose polymers (in its side chains). This plant immunomodulator strongly differ from other plant preparations since the enzymatic fermentation can break down each sort of glycolytic bond except the binding between arabinose and xylose. It results in an arabinoxylan configuration with similar form which exists in the plant retaining its PAMP like properties. Indeed, this gently by hydrolyzing enzyme isolated RBAC has a similar immunomodulatory effect as bacterial PAMP molecules. A great number of publications reports that given in doses between 15 and 45 mg/kg RBAC can activate the type-1 natural effectors, such as phagocytic and NK activities [24-26] and exhibit clinical benefit [27-38].
Statistical analysis
All results are expressed as means +/-SEM. Since STZ caused a considerably decrease in absolute number of peripheral lymphocytes, the changes in various parameters of NK cells were analyzed using only relative evaluations among of total lymphocyte or NK population. At first the Window and Graphpad Instat software package were used for the statistical analysis of experimental data. One-way and repeated measures analysis of variance (ANOVA) was employed. For the relative values a Student’s t and U test according to Wilcoxon, Mann and Whitney using the Statgraphics stastistical package for IBM-compatible computer was also employed.
Present trial was complicated by the fact that STZ at doses that can induce a type-1 diabetes, caused a significant (between 53% and 75%) reduction in the absolute number of peripheral lymphocytes. Concentrations of other blood cell, however, remained normal. Since previous trials reported about a complete bone marrow reconstitution 29-33 days after STZ treatment, we also tried to extend the interval between blood tests and 65 mg/kg STZ application. However, after 60 days the STZ-induced decrease in the number of lymphocytes did not disappear. Therefore, for NK cell investigations we decided to analyze only the relative alterations.
For our in vivo trial three groups, containing six animals in each group, were selected: 1.) negative controls; 2.) rats were treated with 45 mg/kg RBAC 24h prior to blood test but without STZ pretreatment; 3.) 24h before the blood test the animals were also treated with 45 mg/kg RBAC but after 65 mg/kg STX injection which was given nine days previously into the tail vein. Because of STZ-induced lymphopenia we wanted to analyze only the RBAC-induced relative increases compared to the negative control with and without previous STZ treatment. Our work hypothesis was the following: how can act a STZ-induced insulin deficit on the immunomodulator (RBAC)-induced NK responses. To see the differences between the second and third groups better, a single RBAC dose was only given. On base of previous (unpublished) investigations this single dose of RBAC within 24h is yet not able to induce an optimal NK response.
Figure 2 shows using CD161-APC clone the flow cytometric determinations of relative values of NKR-P1 positive cells among the lymphocytes which is the most important marker for rat NK cells. The BRAC-induced increases were related to the negative control. As shown, RBAC without STZ treatment revealed a not significant 13.5% (+/- 6) increase. However, this enhancement was 35.7% (+/-11) and significant (p<0.05) in the STZ-treated group indicating an enhancing effect of insulin deficit on NK frequency.
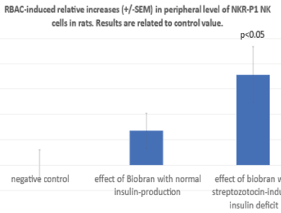
Figure 2. Using CD161-APC clone flow cytometric determinations of RBAC-induced relative percentage increases of NKR-P1 positive NK cells among the lymphocytes without and with previous STZ treatment
As shown in figure 3, hematological investigations supported these results with NKR-P1 frequency. As it is well known, NK cells are derived from bone marrow and morphologically have the appearance of large granular lymphocytes. They count for 5-15% of all lymphocytes in the circulation. LGL frequency among all lymphocytes in peripheral blood was 11,4% (+/-0.87) in negative control group. RBAC without STZ revealed only a not significant increase to 16.25% (+/-2.7) but RBAC with STZ treatment induced a significant elevation of LGL level with 30.75% (+/-6.5; p<0.05).
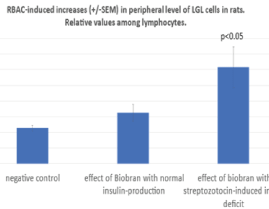
Figure 3. NK cells are derived from bone marrow and morphologically have the appearance of large granular lymphocytes. Percentage of LGL frequency among all lymphocytes in peripheral blood was determined in the three groups of rats
NK cells express a range of activator and inhibitory receptors. Interestingly, many of the inhibitory receptors expressed by NK cells also have an activating counterpart. In the background of activating and inhibitory receptor functions there is a very complex regulatory network. The most important Killing Activator Receptor (KAR) is the NKG2D molecule which is a member of C-type receptor family. NKG2D is a key activator expressed by all circulating NK cells. Therefore, in present study the RBAC-induced relative increases in frequency of NKG2D + - NKR-P1+ cells were also determined using CD161-APC and CD314-PE monoclonal antibodies. As shown in figure 4 the RBAC-induced increases related to negative controls among NK cell population were 10 (+/-6) % without STZ (NS) and 17.4 (+/-7) % with STZ treatment (p<0.05). Similar results were observed if the NKG2D intensity was determined on the NK cells. As shown in figure 5, the RBAC-induced relative increase compared with the negative controls was in NK cell population 12.5% (+/-7) without STZ (NS) and 24.1% (+/-10) after STZ treatment indicating that insulin deficit can has an enhancing effect on RBAC-induced NK response.
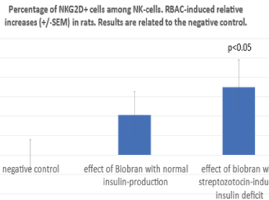
Figure 4. Using CD161-APC and CD314-PE monoclonal antibodies, RBAC-induced relative increases in NKG2 positivity among NKR-P1 cells are shown
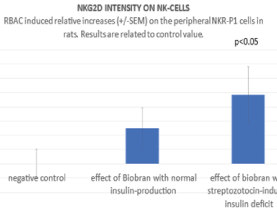
Figure 5. Using CD314-PE the NKG2D intensity was determined on the NKR-P1 cells and compared with negative control
Present results support the hypothesis that the polarity of innate immune system can be associated with the polarity of neuroendocrine system and a disturbed balance in their regulations can also play a role in an increased cancer risk of several patients with diabetes mellitus. As it was mentioned above, insulin also belongs to the growth factors and therefore it appears to be plausible that there is a close relationship between Insulin and the balance between type-1 and type-2 innate immune cells. As shown in figure 1 and mentioned previously, tumor patients parallel with their progression have always stronger predominance of type-2 innate cells. Cytokines of type-2 immune system, such as IL-4 or TGFβ can enhance the insulin sensitivity and are involved of increase in mass, proliferation and differentiation of β cell [17,18,39]. M2 cells secrete miRNA-containing exosomes which improve glucose tolerance and insulin sensitivity in obese mice [40]. In contrast, the type-1 macrophages (M1) and dendritic cells (D1) can take part on the downregulation of sensitivity of insulin receptors resulting in an insulin resistance on the targets [41]. These experiences are consistent with old clinical observations that an acute inflammation, that activates M1 and D1 as well as NK-cells, raises blood glucose level, decreases insulin production by β-cells and reduces the sensitivity of insulin receptors. Therefore, it is not surprising, that the type-1 cytokines, such as IL-1β given into the nucleus accumbens, which belongs to the limbic structures in the medial mediodorsal prefrontal cortex, resulted in an elevated glucose level established by the acute glucose tolerance test [20]. It seems logical that type-1 natural immune defense suppresses the type-2 system which serves to protect insulin production and effect. This insulin-protective effect of type-2 cytokines (such as IL-4 and IL-10) in STZ-induced mice diabetes model was also established [42].
However, we know almost nothing about the effect of insulin on the balance between type-1 and type-2 innate immune mechanisms. Considering that insulin production and efficacy are protected by type-2 immune cells, it seems plausible that insulin, like type-2 system itself, is also involved in inhibiting of type-1 immune defense. Present results support this hypothesis since the responses of NK cells after a single application of an evidence-based and standardized immunomodulator (RBAC) was significant greater in STZ-treated mice. These results indicate that Insulin, belonging to the growth factors, is working together with the type-2 immune cells (such as M2 and D2). The NK-cell activating effect by insulin deficit may help to better understand our previous clinical report in that the inhibition of growth factor receptors by MEK inhibitor revealed astonishing clinical results (rapid complete remissions of brain metastases) if it was combined with M1, D1 and NK-activating RBAC [43]. Since combination of protein kinase signalling pathway inhibitors and natural effector activators could open new perspectives in the tumor therapy, on base of present results it can be so concluded that we must better learn to manipulate the regulatory network both in neuroendocrine and in natural immune system.
The authors thank Viktoria Temesfői for technical help and support this research.
The authors declare that there is no competing or other conflicting interest in relation to this paper.
- Suk S, Kim KW (2019) Diabetes and cancer: cancer should be screened in routine diabetes assessment. Diabetes Metab J 43: 733-743. [Crossref]
- Goto A, Yamai T, Sawada N, Momozawa Y, Kamatani Y, et al. (2020) Diabetes and cancer risk: a Mendelien randomization study. Int J Cancer 146: 712-719. [Crossref]
- Deng L, Gui Z, Zhao L, Wang J, Shen L (2012) Diabetes mellitus and incidence of colorectal cancer: an up-to-dated systematic review and meta-analysis. Dig Dis Sci 57: 1576-1585. [Crossref]
- Sciacca L, Vella V, Frittitta L, Tumminia A, Manzella L, et al (2018) Long acting insulin analogs and cancer. Natur Metab Cardiovasc Dis 28: 436-443. [Crossref]
- Gallagher EJ, LeRoith D (2015) Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol Rev 95: 727-748. [Crossref]
- Ballotari P, Vicentini M, Manicardi V, Gallo M, Ranieri SC, et al. (2017) Diabetes and risk of cancer incidence: results from population-based cohort study in Northern Italy. BMC Cancer 17: 703. [Crossref]
- Vanessa L, Gordon-Dseagu Z, Shelton N, Mindell JS (2013) Epidemiological evidence of relationship between type-1 diabetes mellitus and cancer: a review of the existing literature. Int J Cancer 32: 501-508. [Crossref]
- Van der Schueren B, Ellis D, Faradij RN, Al-Ozairi E, Rosen J, et al. (2021) Obesity in people living with type-1 diabetes. Lancet Diabetes Endocrinol 9: 776-785. [Crossref]
- Liu X-L, Wu H, Zhao L-G, Xu H-L, Zhang W, et al. (2018). Association between insulin therapy and risk of liver cancer among diabetics: a meta-analysis of epidemiological studies. Eur J Gastroenterol Hepatol 30: 1-8. [Crossref]
- Cooke DW, Divall SA, Radovich S (2016) Insulin-like Growth Factors. Williams Textbook Endocrinology, 13th edition, Canada, Elsevier, pp: 977-987.
- Sánchez-Torres C, García-Romo GS, Cornejo-Cortés MA, Rivas-Carvalho A, Sánchez-Schmitz G (2001) CD16+ and CD16- human blood monocyte subsets differentiate in vitro to dentritic cells with different abilities to stimulate CD4+ T cells. Int Immunol 13: 1571-1581. [Crossref]
- Mantovani A (2007) Inflammation and cancer; the macrophage connection. Medicina (Buenos Aires). 67: 32-34. [Crossref]
- Hajto T (2018) New perspectives to improve the MHC-I unrestricted immune mechanisms against malignant tumors. Adv Clin Trans Res 2: 1-13. [Crossref]
- Adamska A, Domenichini A, Falasca M (2017) Pancreas ductal adenocarcinoma: current and evolving therapy. Int J Mol Sci 18: 1336. [Crossref]
- Marcherie D, Conlon K, Bohac GC, Barcenas J, Leslie W, et al. (2012) Effect of pemetrexed on innate immune killer and adaptive T cells in subjects with adenocarcinoma of the pancreas. J Immunother 35: 629-640. [Crossref]
- Trovato R, Fiore A, Sartori S, Cané S, Giugno R, et al. (2019) Immunosuppression by monocytic-myeloid derived suppressor cells in patients with pancreas ductal carcinoma is orchestrated by STAT3. J Immunother 7: 255. [Crossref]
- Chao R, Li D, Yue Z, Huang C, Kou Y, et al. (2020) Interleukin-4 restores insulin sensitivity in insulin resistant osteoblasts by increasing the expression of insulin receptor substrate. Biochamistry (Mosc) 5: 334-343. [Crossref]
- Lee JH, Lee JH, Rane SG (2021) TGF-beta signaling in pancreatic islet beta cell development and function. Endocrinol 162: 233. [Crossref]
- Herlitz-Cifuentes HS, Garces PCF, Fernandez LIL, Guzman-Gutierrez EA (2015) Effect of systematic inflammation on the function of insulin and glucose metabolism in rheumatoid arthritis. Curr Diabetes Rev 12: 156-162. [Crossref]
- Takács G, Papp S, Lukáts B, Szalay C, Nagy B, et al. (2010) Homeostatic alteration after IL-1beta microinjection into the nucleus accumbens of the rat. Appetite 54: 354-362. [Crossref]
- Ganda OP, Rossini AA, Like AA (1976) Studies on streptozotocin diabetes. Diabetes 25: 595-603. [Crossref]
- Szkudelski T (2001) The mechanism of alloxan and streptozotocin action in beta cells of the pancreas. Physiol Res 50: 537-546. [Crossref]
- Nagy B, Szabó I, Takács G, Csetényi B, Hormay E, et al. (2016) Impaired glucose tolerance after streptozotocin microinjection into the mediodorsal prefrontal cortex of the rat. Physiol Int 103: 403-412. [Crossref]
- Cholujova D, Jakubikova J, Sedlak J (2009) BioBran-augmented maturation of human monocyte-derived dendritic cells. Neoplasma 56: 89-95. [Crossref]
- Tan BL, Norhaizan ME (2017) Scientific evidence of rice by-products for cancer prevention: chemopreventive properties of waste products from rice milling on carcinogenesis in vitro and in vivo. Biomed Res Int 2017: 9017902. [Crossref]
- Pérez-Martínez A, Valentín J, Fernández L, Hernández-Jiménez E, López-Collazo E, et al. (2015) Arabinoxylan rice bran (MGN-3/Biobran) enhances natural killer cell-mediated cytotoxicity against neuroblastoma in vitro and in vivo. Cytotherapy 17: 601-612. [Crossref]
- Badr El-Din NK, Ali DA, El-Dein MA, Ghoneum M (2016) Enhancing the apoptotic effect of a low dose of paclitaxel on tumor cells in mice by arabinoxylan rice bran (MGN-3/Biobran). Nutr Cancer 68: 1010-1020. [Crossref]
- Badr El-Din NK, Abdel Fattah SM, Pan D, Tolentino L, Ghoneum M (2016) Chemopreventive activity of MGN-3/Biobran against chemical induction of glandular stomachcarcinogenesis in rats and its apoptotic effect in gastric cancer cell. Integr Cancer Ther 15: NP26-NP34. [Crossref]
- Ghoneum M, Agrawal S (2014) Mgn-3/biobran enhances generation of cytotoxic D8+ T cells via upregulation of dec-205 expression on dendritic cells. Int J Immunopathol Pharmacol 27: 523-530. [Crossref]
- Ghoneum M, Badr El-Din NK, Ali DA, El-Dein MA (2014) Modified arabinoxylan from rice bran, MGN-3/biobran, sensitizes metastatic breast cancer cells to paclitaxel in vitro. Anticancer Res 34: 81-87. [Crossref]
- Ghoneum M, Badr El-Din NK, Abdel Fattah SM, Tolentino L (2013) Arabinoxylan rice bran (MGN-3/Biobran) provides protection against whole-body -irradiation in mice via restoration of hematopoietic tissues. J Radiat Res 54: 419-429.
- Ghoneum M, Agrawal S (2011) Activation of human monocyte-derived dendritic cells in vitro by the biological response modifier arabinoxylan rice bran (MGN-3/Biobran). Int J Immunopathol Pharmacol 24: 941-948. [Crossref]
- Badr El-Din NK, Noaman E, Ghoneum M (2008) In vivo tumor inhibitory effects of nutritional rice bran supplement MGN-3/Biobran on Ehrlich carcinoma-bearing mice. Nutr Cancer 60: 235-244. [Crossref]
- Gollapudi S, Ghoneum M (2008) MGN-3/Biobran, modified arabinoxylan from rice bran, sensitizes human breast cancer cells to chemotherapeutic agent, daunorubicin. Cancer Detect Prev 32: 1-6. [Crossref]
- Bang MH, Riep TV, Thinh NT, Song LH, Dung TT, et al. (2010) Arabinoxylan rice bran (MGN-3) enhances the effect of interventional therapies for the treatment of hepatocellular carcinoma: A three-year rabdomized clinical trial. Anticancer Res 30: 5145-5152. [Crossref]
- Itoh Y, Mizuno M, Ikeda M, Nakahara R, Kubota S, et al. (2015) A randomized, double blind pilot trial of hydrolyzed rice bran versus placebo for radioprotective effect on acute gastroenteritis secondary to chemoradiotherapy in patients with cervical cancer. Evid Based Complement Alternat Med 2015: 974390. [Crossref]
- McDermott C, Richards SC, Thomas PW, Montgomery J, Lewith G (2006) A placebo controlled, double-blind, randomized controlled trial of a natural killer cell stimulant (BioBran MGN-3) in chronic fatigue syndrome. QJM 99: 461-468. [Crossref]
- Nichols WK, Vann LL, Spellman JB (1981) Streptozotocin effect on T lymphocytes and bone marrow cells. Clin Exp Immunol 46: 627-632.
- Chen Z, Qin X, Zhang X, Liu B, Chen M (2020) Upregulation of IL-4 signaling contributes to aerobic exercise-induced insulin sensitivity. Biochem Biophys Res Commun 525: 662-667.
- Ying W, Gao H, Gomes DosReis FC, Bandyopadhyay G, Ofrecio JM, et al. (2021) MiR-690, an exosomal-derived miRNA from M2 polarized macrophages, improves insulin sensitivity in obese mice. Cell Metab 33: 781-793. [Crossref]
- Herlitz-Cifuentes HS, Garces PCF, Lamperti-Fernandez PC, Guzman-Gutierrez EA (2015) Effect of systematic inflammation ont he function of insulin and glucose metabolism in rheumatoid arthritis. Curr Diabetes Rev 12: 156-62. [Crossref]
- Preisser TM, Da Cunha VP, Santana MP, Pereira VB, Cara DC, et al. (2021) Recombinant Lactococcus lactis carrying IL-4 and IL-10 coding vectors protects against type-1 iabetes of NOD mice and attenuates insulitis in the STZ-induced model. J Diabetes Res 2021: 6697319.
- Hajto T (2017) Can a standardized plant immunomodulator (rice branarabinoxylan concentrate/MGN-3) increase the effects of MEK and BRAF inhibitors with clinical benefit? Case report of a patient with carcinoma in biliary duct. Res Rev Insights 1: 1-4.





