Aim: To determine whether there were ethnic differences in HbA1c concentrations in adults with normal and abnormal glucose tolerance.
Methods: Cross-sectional data were from 3,559 participants not previously diagnosed with diabetes aged 35-74 years. Participants underwent a 75 gm oral glucose tolerance test. Glucose and fructosamine levels were determined enzymatically, and HbA1c by cation exchange high performance liquid chromatography.
Results: Compared with Europeans, and after adjusting for age and gender, HbA1c levels were on average 5.5 (se=0.25) mmol/mol higher for Pacific, 2.5 (0.24) mmol/mol higher for Māori, and 2.3 (0.40) mmol/mol higher for Asians. These ethnic differences were attenuated in a multivariable regression model for HbA1c, mainly due to the inclusion of current smoking habit and BMI, but still retained statistical significance. HbA1c levels were higher in Māori, Pacific and Asian participants with normal glycaemia, Māori and Pacific people with impaired fasting glycaemia, and impaired glucose tolerance, and Pacific people with newly diagnosed diabetes compared to Europeans after adjusting for age, gender, fasting and 2 hour glucose. Ethnic differences of HbA1c concentrations increased with increasing glycaemia compared to Europeans. After adjusting for age, gender and body mass index, the squared semi-partial correlations for HbA1c were 50.5% for fasting glucose and 3.6% for 2 hour glucose.
Conclusions: HbA1c concentrations in this population mainly reflected fasting glucose levels. The higher HbA1c level for the same degree of glycaemia in non-European ethnic groups has clinical relevance for both diagnosis and management.
Mori and Pacific people living in New Zealand have a higher prevalence of Type 2 diabetes mellitus (DM) compared to Europeans 1,2. They are more likely to develop renal disease and renal failure requiring dialysis 3-5 and the prevalence of diabetic retinopathy is higher compared to Europeans 3,5,6.
Ethnic differences in mean glycated haemoglobin (HbA1c) levels have been previously described in people without diabetes in the U.S. 7-10 and in people with impaired glucose tolerance or diabetes 11,12. Ethnic differences have been reported that account for variation in HbA1c of up to 4 mmol/mol 11 for similar levels of glycaemic control. While intra-individual biological variation of HbA1c is small and changes little over time, inter-individual variation between patients, for the same degree of glycaemic control, is much larger 13,14. Possible explanations include subtle differences in entry of glucose into red blood cells 15 and varying red cell lifespan in the circulation 16,17. Genome-wide association analysis shows that only a minority of loci related to raised HbA1c clearly relate to glucose metabolism, so a number of other factors, both physiological and potentially pathological, are likely to be involved 18.
Since HbA1c is now regarded as either the preferred [19] or acceptable alternative [20] means of diagnosing diabetes, its use to diagnose diabetes can affect population prevalence and which individuals are diagnosed with diabetes in different ethnic groups [21]. Inter-individual variation in the rate of HbA1c formation also has management implications, for example on rates of adverse events, as suggested by a recent post-hoc analysis of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial [22]. These inter-individual and inter-ethnic differences therefore have significant clinical, as well as resource and health-care cost, implications. The purpose of this study was to examine whether there were ethnic differences in HbA1c levels in a New Zealand population.
Subjects
The Auckland Diabetes, Heart and Health Survey was carried out between December 2001 and November 2003 in 4,049 randomly sampled adults aged 35 to 74 years (response rate 65%). A more detailed description of the sampling method is provided in Sundborn et al. [2]. Adults with missing HbA1c results (n=27) and previously diagnosed diabetes (n=463) were excluded leaving 3,559. Participants comprised 47.8% males and 52.2% females, 46.3% Europeans, 24.8% Māori, 22.0% Pacific and 6.9% Asian people. Asian people comprised 20.8% Indian (South Asian) and 79.2% from China, Hong Kong, Korea, Taiwan and the Philippines.
Interviews were carried out in community venues or clinics close to participant’s homes. Personnel were trained in the administration of the questionnaires and in taking blood pressure and other measurements. Ethnicity was classified according to the 2006 NZ census [23]. Around 80% of New Zealanders can claim some British ancestry and an estimated 17% are entitled to British passports [23]. The European (white Caucasian) ethnic group also included other white ethnicities such as Caucasian people from South African and the United States of America.
Approval and Consent
The New Zealand Ministry of Health Auckland Ethics Committees granted ethnical approval (NTX/12/EXP/008/AM03). Written informed consent was obtained from all subjects.
Blood collection
Participants fasted from 10pm the evening before the interview. A 75 gm oral glucose tolerance test (oGTT) was carried out in all participants and a fasting and 2 hour post 75gm glucose challenge (Glucaid drink) samples were collected for glucose measurements.
Laboratory measurements
Plasma glucose was measured using an enzymatic method (Glucose oxidase Roche (NZ)). Categorisation of glucose tolerance status was evaluated by 1998 WHO criteria (using fasting glucose ≥ 7.0 mmol/L and/or 2 h post glucose load of ≥ 11.1 mmol/l for diabetes, fasting glucose < 7.0 mmol/L and 2 h glucose between 7.8 to 11.0 mmol/l for impaired glucose tolerance (IGT) and fasting glucose of 6.1 to 6.9 mmol/L for impaired fasting glucose (IFG) [24]. Serum total cholesterol and (direct) HDL-cholesterol were measured using standard colourimetric autoanalyser methods (Roche Hitachi NZ). After phlebotomy, glucose samples were stored on ice and centrifuged within 1 hour of collection.
Haemoglobin A1c (HbA1c) was measured by cation exchange high performance liquid chromatography (Biorad Variant II). The in-house inter-batch coefficients of variation for low control material were glucose 2.1%, HbA1c 1.7%, fructosamine 2.0%, cholesterol 1.4% and HDL-cholesterol 1.2%; those of abnormal (high) control were glucose 1.3%, HbA1c 2.1%, fructosamine 1.9%, cholesterol 1.2%, and HDL-cholesterol 2.7%. The laboratory maintained ongoing acceptable performance in the Royal Australasian College of Pathologists (RCPA) external proficiency QAP programme for all tests and continuous accreditation against ISO15189.
Other measurements
Weight and height were measured to the nearest 0.1 kg and 0.5 cm, respectively. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m). An Omron-Hem-706 oscillometric blood pressure monitor was used to measure systolic and diastolic blood pressure twice (5 minutes apart) after at least 15 minutes in the sitting position.
Data analysis
The characteristics of participants by ethnicity were compared using analysis of covariance for normally distributed variables and the χ2 test for categorical variables. Independent correlation coefficients were compared using Fisher’s Z transformation [25]. Multivariable regression was used to determine whether ethnic differences in HbA1c could be explained by concentrations of other variables. Statistical analyses were carried out using SAS version 9.4 [26].
Table 1 shows the characteristics of participants by ethnicity. Compared to Europeans, Māori, Pacific and Asians were of lower age and had lower total- and HDL-cholesterol concentrations and higher fasting and 2 hour glucose, HbA1c, and diastolic blood pressure levels. BMI levels were higher in Pacific and Māori participants and lower in Asians compared to Europeans, systolic blood pressure was higher in Pacific and lower in Asian participants, and current smoking levels were higher in Māori and Pacific adults. Prevalence of newly diagnosed diabetes was significantly higher in all three non-European groups. Pacific people had a higher prevalence of impaired fasting glycaemia (IFG).
Table 1. Mean (SD) or percent for characteristics of participants by ethnicity.
|
European |
Māori |
Pacific |
Asian |
Number |
1,646 |
881 |
784 |
248 |
Male gender |
49.5% |
43.4%** |
48.9%† |
48.8% |
Age (years) |
55.1 (11.27) |
52.4 (11.57)*** |
49.6 (11.49)*** ††† |
49.2 (10.93)*** ††† ‡‡‡ |
BMI (kg/m2) |
27.3 (4.53) |
30.1 (6.14)*** |
33.9 (6.10)*** ††† |
24.8 (3.36)*** ††† |
Fasting glucose (mmol/L) |
5.1 (0.64) |
5.62 (0.81)** |
5.4 (1.26)*** ††† |
5.3 (0.97)*** ‡ |
2 hour glucose (mmol/L) |
5.6 (1.97) |
6.0 (2.41)*** |
6.0 (2.81)*** |
6.3 (2.97)*** ‡ |
HbA1c (mol/mol) |
37.1 (4.40) |
39.1 (5.97)*** |
41.7 (8.09)*** ††† |
38.5 (7.31)*** ‡‡‡ |
Cholesterol (mmol/L) |
5.6 (1.02) |
5.5 (1.02)** |
5.4 (1.02)*** † |
5.4 (1.07)*** |
HDL-cholesterol (mmol/L) |
1.49 (0.41) |
1.39 (0.36)*** ‡‡‡ |
1.30 (0.31)*** ††† |
1.41 (0.36)** ‡‡‡ |
Systolic BP (mm Hg) |
123.5 (20.10) |
124.5 (22.03) |
127.3 (22.04)*** †† |
120.6 (2.89)* † ‡‡‡ |
Diastolic BP (mm Hg) |
75.0 (9.84) |
78.0 (12.15)*** |
79.8 (12.10)*** ††† |
76.2 (10.74) ‡‡‡ |
Current smoker |
12.2% |
28.8%*** |
24.0%*** † |
9.7%†††‡‡‡ |
Impaired fasting glucose |
2.9% |
3.5% |
5.1%** |
3.2% |
Impaired glucose tolerance |
9.2% |
11.2%* |
10.5% |
8.9% |
Newly diagnosed diabetes |
2.6% |
4.0%* |
5.1%** †† |
3.2% |
* 0.01 ≤ P < 0.05; ** 0.001 ≤ P < 0.01; *** P < 0.001 compared to Europeans. † 0.01 ≤ P < 0.05; †† 0.001 ≤ P < 0.01; ††† P < 0.001 compared to Maori. ‡ 0.01 ≤ P < 0.05; ‡‡ 0.001 ≤ P < 0.01; ‡‡‡ P < 0.001 compared to Pacific.
Correlations between HbA1c and fasting and 2 hour glucoses in the study group were highly significant (r=0.70 and 0.60, respectively). For each ethnic group they were: Europeans r=0.47 and 0.44; Māori r=0.68 and 0.59; Pacific r=0.84 and 0.73; and Asians r=0.79 and 0.75, respectively. After adjusting for age, gender and body mass index, the proportion of the HbA1c variance explained in the study population was 50.5% by fasting glucose and 3.6% by 2 hour glucose when both were entered into the multivariable model.
Table 2 shows the squared semi-partial correlation coefficients for HbA1c in the multivariable model for fasting and 2 hour glucose levels by ethnicity and diabetes status after adjusting for age and gender. Apart from IFG in Europeans and Asians, the contribution of fasting glucose dominated the 2 hour glucose contribution. The squared semi-partial correlation coefficients for HbA1c were highest in those with newly diagnosed diabetes (Table 2) followed by those with IGT (with the exception of Māori in whom both fasting and 2 hour squared partial correlations were higher for IFT).
Table 2. Squared semi-partial correlations (in %) for HbA1c explained by fasting and 2 hour glucose levels by ethnicity and diabetes status after adjusting for age and gender.
|
European |
Māori |
Pacific |
Asian |
|
FPG 2hrPG |
FPG 2hrPG |
FPG 2hrPG |
FPG 2hrPG |
All |
21.7% 5.8% |
47.2%*** 3.5% |
70.8%*** 2.7% |
63.3%*** 6.0% |
NGT |
8.4% 1.4% |
8.5% 2.6% |
16.2%** 1.4% |
9.7% 3.2% |
IFG |
0.07% 0.6% |
20.9% *** 0.02% |
15.6%*** 7.4%*** |
4.7%*** 6.5%*** |
IGT |
7.5% 4.0% |
17.0% *** 2.7% |
25.5%*** 5.2% |
29.8%*** 9.2% |
New DM |
32.4% 24.4% |
78.1%*** 0.4%*** |
83.8%*** 3.6%*** |
89.5%*** 5.8%*** |
FPG = fasting plasma glucose; 2hrPG = 2 hour post glucose load glucose; all = total in ethnic group; NGT = normal glucose tolerance; IFG = impaired fasting glucose; IGT = impaired glucose tolerance; New DM = newly diagnosed diabetes. * 0.01 < P < 0.05; ** 0.001 < P < 0.01; *** P < 0.001 compared to Europeans.
Glycaemic measures by diagnostic category and ethnicity are shown in Table 3. Fasting and 2 hour glucose levels were higher in Māori and Asian people with normal glycaemia (NG), but only 2 hour results were significantly higher in Māori and Asian with newly diagnosed diabetes compared to Europeans, respectively. No significant differences for fasting and 2 hour glucoses were seen for Māori and Asian with intermediate degrees of glucose intolerance (IFG, IGT). By contrast, Pacific adults had slightly higher fasting glucose at all degrees of glycaemia, but no significant differences for 2 hour glucose results.
With the exception of newly diagnosed diabetes, Māori with NG, IFG and IGT had higher HbA1c levels compared to Europeans, which remained after further adjusting for fasting and 2 hour glucose results (Table 3). Pacific adults also had higher HbA1c levels compared to Europeans, both before and after adjusting for fasting and 2 hour glucoses concentrations in all glycaemic groups. Asians had higher HbA1c levels compared to Europeans in adults with normal glycaemia and with newly diagnosed diabetes, but that only remained significant in the normal glycaemic group after further adjusting for fasting and 2 hour glucose levels.
Table 3. Mean (se) glycaemic measures by ethnicity and diabetes status adjusted for age and gender.
|
European (n=1,646) |
Māori (n=881) |
Pacific (n=784) |
Asian (n=248) |
Normal glycaemia |
|
|
|
|
Fasting glucose (mmol/L) |
4.9 (0.01) |
5.0 (0.02)** |
5.1 (0.02)*** |
5.1 (0.03)** |
2 hr glucose (mmol/L) |
5.0 (0.03) |
5.3 (0.05)*** |
5.0 (0.05) |
5.5 (0.09)*** |
HbA1c (mmol/mol) |
36.0 (0.10) |
38.0 (0.14)*** |
39.8 (0.15)*** |
37.3 (0.26)*** |
Adj HbA1c (mmol/mol) |
36.2 (0.10) |
37.9 (0.13)*** |
39.7 (0.15)*** |
37.1 (0.25)** |
Fructosamine (µmol/L) |
230.3 (0.46) |
229.3 (0.61) |
231.0 (0.71) |
237.0 (1.21)*** |
Adj Fructosamine (µmol/L) |
230.2 (0.47) |
229.4 (0.65) |
230.6 (0.73) |
236.9 (1.24)** |
Impaired fasting glucose |
Fasting glucose (mmol/L) |
6.3 (0.03) |
6.3 (0.04) |
6.4 (0.03)* |
6.4 (0.08) |
2 hr glucose (mmol/L) |
5.7 (0.19) |
5.8 (0.22) |
6.0 (0.20) |
6.0 (0.45) |
HbA1c (mmol/mol) |
39.7 (0.66) |
42.0 (0.81)* |
45.4 (0.71)*** |
40.9 (1.64) |
Adj HbA1c (mmol/mol) |
40.0 (0.65) |
41.9 (0.75)* |
44.7 (0.70)*** |
40.5 (1.53) |
Fructosamine (µmol/L) |
237.6 (2.46) |
235.9 (3.00) |
241.7 (2.64) |
247.8 (6.12) |
Adj Fructosamine (µmol/L) |
238.5 (2.44)) |
235.8 (2.84) |
239.1 (2.63) |
245.8 (5.80) |
Impaired glucose tolerance |
Fasting glucose (mmol/L) |
5.5 (0.05) |
5.5 (0.06) |
5.7 (0.07)** |
5.7 (0.13) |
2 hr glucose (mmol/L) |
8.9 (0.07) |
8.9 (0.09) |
8.9 (0.10) |
8.9 (0.19) |
HbA1c (mmol/mol) |
40.1 (0.41) |
42.2 (0.49)** |
45.4 (0.56)*** |
41.2 (1.05) |
Adj HbA1c (mmol/mol) |
40.4 (0.37) |
42.3 (0.44)*** |
44.9 (0.50)*** |
40.9 (0.94) |
Fructosamine (µmol/L) |
234.4 (1.58) |
234.8 (1.91) |
242.2 (2.17)** |
242.5 (4.07) |
Adj Fructosamine (µmol/L) |
234.8 (1.56) |
235.0 (1.87) |
241.4 (2.15)* |
241.9 (4.00) |
Newly diagnosed diabetes |
Fasting glucose (mmol/L) |
7.1 (0.36) |
7.5 (0.40) |
8.2 (0.31)* |
7.5 (0.63) |
2 hr glucose (mmol/L) |
11.8 (0.65) |
14.1 (0.73)* |
12.3 (0.55) |
15.4 (1.12)** |
HbA1c (mmol/mol) |
45.5 (2.38) |
52.1 (2.60) |
56.8 (2.00)*** |
55.1 (4.13)* |
Adj HbA1c (mmol/mol) |
49.3 (1.03) |
52.0 (1.17) |
55.7 (0.90)*** |
52.8 (1.83) |
Fructosamine (µmol/L) |
254.2 (8.22) |
275.2 (8.98) |
276.6 (6.90)* |
305.1 (14.25)** |
Adj Fructosamine (µmol/L) |
266.3 (4.46) |
274.8 (5.67) |
272.2 (3.92) |
298.7 (7.94)*** |
Adj HbA1c and Adj Fructosamine are further adjusted for fasting and 2 hour glucose levels. * 0.01 < P < 0.05; ** 0.001 < P < 0.01; *** P < 0.0001 compared to Europeans.
Compared to Europeans, fructosamine levels were higher in Asians with normal glycaemia and newly diagnosed diabetes, and in Pacific with IGT, and newly diagnosed diabetes (Table 3). These differences remained significant after further adjusting for fasting and 2-hour glucose levels with the exception of Pacific people with newly diagnosed diabetes.
In the study population, HbA1c differences (adjusted for age and gender) for different ethnicities compared with Europeans were +5.5 (se=0.25) mmol/mol for Pacific, +2.5 (0.24) mmol/mol for Māori, and +2.3 (0.40) mmol/mol in Asians. After further adjusting for fasting and 2 hour glucose in the total population, HbA1c differences were on average +3.6 (se=0.18) mmol/mol for Pacific, +2.5 (0.17) mmol/mol for Māori, and +0.8 (0.28) mmol/mol for Asians compared with Europeans. When these ethnic groups were included in the multivariable regression model as dummy variables (with Europeans as the reference group) adjusted for all other variables in the model these differences were attenuated (Table 4) with the exception of Asians, but still retained statistical significance. The attenuation of these ethnic differences in HbA1c was mainly due to the inclusion of current smoking and body mass index. In the multivariant model, Pacific people showed the greatest difference compared to Europeans, followed by Māori and then Asians. The significance levels were unchanged after further adjusting for fructosamine concentrations. Substitution of waist for BMI did not change the conclusions, but the t-value for BMI was greater in both normal glycaemic people and the total study population. Total energy, carbohydrate and alcohol intakes were not related to HbA1c, nor was physical activity (individual data not shown). The inclusion of interaction terms between each ethnic group and smoking were not statistically significant.
Figure 1 shows the relationship between HbA1c and mean plasma glucose (average of fasting and 2 hour glucose concentrations) concentrations in each ethnic group (using a quadratic regression model). The differences between the European line and Asian, Māori, and Pacific lines increased as mean plasma glucose levels increased, with the greatest difference in Pacific adults. These regression models explained the highest percentage variation of HbA1c in Asians (70%), followed by Pacific (69%), Māori (49%) and Europeans (26%) (Figure 1).
This study shows that HbA1c concentrations are higher in Mori, Pacific and Asian adults compared to Europeans with both normal and abnormal glycaemia. These higher HbA1c concentrations persisted after adjustment for fasting and 2 hour glucose concentrations, but were mainly attenuated by current smoking and BMI (). Further adjustment for fructosamine levels, another marker of glycation outside of the red cell, did not change these significant differences. Compared to Europeans, Maori and Pacific participants had higher mean body mass indices and had significantly more current smokers, whereas Asians had a lower mean body mass index and a lower prevalence of smokers compared to Europeans ().
Our study adds to the growing evidence for interethnic differences in the rate of HbA1c formation for the same level of glycaemia. In U.S. adults without diabetes, HbA1c levels were higher overall in African American men and women compared to non-Hispanic whites [27]. Another study in the US combined two cross-sectional studies and reported that black ethnicity in adults without known diabetes was associated with a 2 mmol/mol higher HbA1c level in the Screening for Impaired Glucose tolerance study and 3 mmol/mol higher in NHANES III when estimated using a multivariable regression model that included gender, BMI, haemoglobin, level of education, fasting and 2 hour glucose levels [28]. This is similar to the higher levels of 3.1 mmol/mol in Pacific, 1.2 mmol/mol in Māori and 1.2 mmol/mol in Asians compared to Europeans in our own fully adjusted model in adults without known diabetes (Table 4; All) and 3.9 mmol/mol in Pacific, 1.6 mmol/mol in Māori and 1.7 mmol/mol in Asians compared to Europeans in the fully adjusted model in adults without abnormal glycaemia (individual data not shown). Furthermore, ethnic differences of HbA1c concentrations increased with increasing glycaemia compared to Europeans (Figure 1).
Table 4. Multivariable regression model with HbA1c as dependent variable in participants with normal glycaemia (n=2,927) and study population (n=3,559).
|
Normal glycaemia |
All |
Variable |
β-coefficient (se) |
P-value |
Partial R2 |
β-coefficient (se) |
P-value |
Partial R2 |
Māori |
1.27 (0.17) |
<0.0001 |
0.4% |
1.22 (0.17) |
<0.0001 |
0.2% |
Pacific |
2.80 (0.19) |
<0.0001 |
7.7% |
3.13 (0.20) |
<0.0001 |
8.0% |
Asian |
1.44 (0.27) |
<0.0001 |
0.2% |
1.16 (0.28) |
<0.0001 |
0.3% |
Age (years) |
0.11 (0.01) |
<0.0001 |
10.9% |
0.08 (0.01) |
<0.0001 |
6.8% |
Fasting glucose (mmol/L) |
2.04 (0.16) |
<0.0001 |
6.7% |
3.62 (0.10) |
<0.0001 |
41.7% |
2 hr glucose (mmol/L) |
0.23 (0.06) |
<0.0001 |
0.4% |
0.58 (0.04) |
<0.0001 |
2.8% |
BMI (kg/m2) |
0.10 (0.01) |
<0.0001 |
1.4% |
0.07 (0.01) |
<0.0001 |
0.2% |
Serum cholesterol (mmol/L) |
0.36 (0.07) |
<0.0001 |
0.9% |
0.27 (0.07) |
<0.0001 |
0.2% |
Current smoker |
1.51 (0.17) |
<0.0001 |
1.9% |
1.73 (0.18) |
<0.0001 |
1.1% |
Male sex |
0.28 (0.14) |
0.0376 |
0.1% |
-0.10 (0.14) |
0.4748 |
0.0% |
R2 = 30.7 % of the variation explained for normal glycaemia and 61.3% for study group.
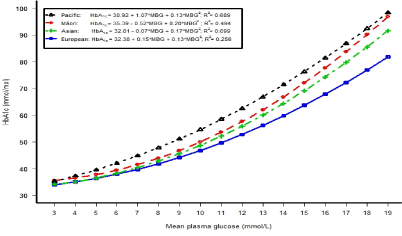
Figure 1. The relationship between HbA1c and mean plasma glucose (average of fasting and two hour glucose concentrations) concentrations in each ethnic group (using a quadratic regression model).
A U.K. study of adults without known diabetes, reported that HbA1c and fructosamine levels were higher in Black than in White adults, even though fasting glucose levels were similar [29]. By contrast, in the normoglycaemic group in the current study some of the ethnic differences were explained by higher glucose concentrations in Māori, Pacific and Asians compared to Europeans, nevertheless the significant differences in HbA1c persisted after glucose adjustment (Tables 3 and 4). Fructosamine levels were higher only in Asians with normal glycaemia and newly diagnosed diabetes that remained significant after adjusting for fasting and 2 hour glucose levels (Table 3).
Another U.S. meta-analysis that was carried out in adults with impaired glucose tolerance reported that adjusted HbA1c levels were highest in African Americans, followed by American Indians, Asians, and Hispanics and lowest in non-Hispanic whites [12]. In the current study, HbA1c levels in participants with impaired glucose tolerance were higher in Pacific, followed by Māori and Asians than in Europeans (Table 3).
A U.S study in adults without diabetes reported significant associations between glycated haemoglobin and older age, male sex, non-Hispanic black ethnicity, hypercholesterolaemia, higher BMI, and lower attained education [30]. HbA1c has also been reported to be correlated with smoking and clinically overt atherosclerosis, but not caloric intake, physical activity or alcohol intake in a U.S. population [13], as was observed here (individual data not shown). These were similar to the associations with HbA1c reported in Table 3 in normoglycaemic patients. However, educational attainment failed to reach significance in the current study, and we did not have the ability to measure clinically overt atherosclerosis.
While pathological states leading to a shortened red cell lifespan (e.g. haemolysis, venesection, chronic blood loss) are well recognised to cause misleadingly low HbA1c levels, some evidence suggests that the inter-individual and possibly ethnic variation in HbA1c levels may be at least be partly due to differences in the mean age of circulating red cells even in otherwise healthy patients with no haematological evidence of any pathology [31]. Inter-individual differences have also been described in the entry of glucose into red cells, and the steady state intracellular concentration of glucose compared with plasma [16].
A small clinical study has shown that only 19 to 48% of the variance in glycated haemoglobin in normal glycaemic individuals was explained by fasting or 2 hour post glucose load glucose levels [14]. In the current study, the proportion of the HbA1c variance explained was 50.5% for fasting glucose and 3.6% for 2 hour glucose after adjusting for age and gender. In a small study (of 12 people), 85% of the total variance was due to inter-individual variation, 6% from intra-individual variation, and the remaining 9% from assay variation [29]. It has been hypothesised that ethnic differences may be due to differences in glycation, erythrocyte survival, biological differences, types of lifestyles, health care access and utilization, quality of care, or socioeconomic factors [9,32]. However, heritability studies indicate that there is a significant genetic contribution [33,34], including a majority of genetic loci that appear unrelated to glucose metabolism [34].
When comparing traditional glucose criteria with HbA1c, Christensen [35] noted a wide variation between different countries and different studies in rates of diagnosis. While some of these differences may be related to study methodologies [35], putative biological explanations also exist. These may include differences in rates of haemoglobinopathy (e.g. higher rates of thalassemia in Africans and Southeast Asians) and nutritional iron deficiency (e.g. higher rates in East Indians). The prevalence of haemoglobinopathies in New Zealand is unknown. Differences between ethnic groups in the frequency of polymorphisms of multiple genetic loci may also contribute [34,36].
On a population basis, these findings have significant potential healthcare resource implications for rates of diagnosis of diabetes. Even in a relatively homogeneous European population in the A1c-Derived Average Glucose (ADAG) study [37], there was significant variation in the relationship between mean glucose levels and HbA1c and this variation was greater still when ethnic differences are considered. In view of the potential for HbA1c results to misclassify patients [36], sole reliance on HbA1c as a diagnostic criterion has been discouraged by expert groups especially in those in whom the possibility of an erroneous result is increased [19,20].
Inter-individual and inter-ethnic differences may also have relevance to the monitoring of patients, especially those in whom tight control is desired. Differences in glycation rates tend to be consistent for an individual patient and will bias the clinical assessment to be relatively favourable (for those with low glycation rates; i.e. low ‘haemoglobin glycation index’) or unfavourable (for those with high glycation rates; i.e. high ‘haemoglobin glycation index’), especially if not considered carefully along with glucose results [18]. Hempe [18] showed that in a multi-ethnic population of young patients with Type 1 DM, about 30% of patients had HbA1c results that were statistically significantly higher or lower compared with the overall relationship for that population, potentially leading to errors in management. A retrospective analysis of the ACCORD study, this time in patients with Type 2 DM [22], also showed that in tightly controlled patients hypoglycaemic events were more likely in those forming HbA1c more quickly (i.e. high haemoglobin glycation index), perhaps by misleading caregivers into believing that mean glucose levels were higher than they truly were.
Some evidence shows that a faster rate of glycation (high glycation index) is associated with a greater prevalence of retinopathy, nephropathy and mortality [38]. The higher rate of complications in non-European ethnic groups such as Māori and Pacific Island patients is well known [3-6]. It is therefore possible that this association may reflect not only ethnic differences in glucose control, but also different rates of glucose entry and attachment to proteins in multiple tissues (for which faster HbA1c formation in red cells may be a coincidental but clinically relevant surrogate).
One study reported that the better association between HbA1c and fasting glucose could be related to the fact that HbA1c correlated better with fasting glucose in the high range and with 2 hour glucose levels in the low range [39]. However, we did not observe the same association as fasting glycaemia explained a higher proportion of the variation in HbA1c at all categories of glucose tolerance (apart from Europeans and Asians with IFG: Table 2). Black adults living in the U.S have been reported as having higher fructosamine levels compared to white persons [7]. In the current study, only Asians in the normal glycaemic and newly diagnosed diabetes groups had significantly higher mean fructosamine levels compared to Europeans. We could find no reports of higher fructosamine levels in Asians.
Strengths of the work include an important clinical research topic, and the inclusion of multiple ethnic groups studied using the same methods, as well as the random selection of participants and a relatively high response rate of 65% into the study. However, the cross-sectional design of the study does not allow examination of the effect of duration. Another limitation is the relatively small sample size of Asians, which may have reduced the study’s ability to detect differences between this group and Europeans. Further limitations include only one measurement of the glycaemic measures.
Compared to Europeans and after adjusting for fasting and 2 hour glucose concentrations HbA1c levels were higher in Māori, Pacific and Asians participants without diabetes, Māori and Pacific people with IFG and IGT, and Pacific people with newly diagnosed diabetes. Although attenuated after adjusting for variables known to be associated with HbA1c levels, the positive bias for HbA1c formation remained significant in Māori, Pacific and Asian participants. HbA1c levels were more highly associated with fasting glucose than 2 hour glucose. Furthermore, ethnic differences of HbA1c concentrations increased with increasing glycaemia compared to Europeans.
2021 Copyright OAT. All rights reserv
HbA1c concentrations in this population mainly reflected fasting glucose levels. The higher HbA1c level for the same degree of glycaemic control in non-European ethnic groups has clinical relevance for both diagnosis and management.
CK, PM and RJ conceptualised and designed the study. CK collected the data. PM analysed the data with input from CK, TK, GS and RJ. All authors were involved in data interpretation. PM and CK drafted the manuscript and all authors critically revised the manuscript. All authors approved the final submitted version and agreed to be accountable for the manuscript.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Written informed consent was obtained from all individual participants included in the study.
This work was supported by a grant from the Health Research Council (HRC) of New Zealand (Grant number: 12/223). Grant applicants were: PA Metcalf, T Keneally, C Kyle, RT Jackson. The HRC had no involvement with the study design, data collection, and analysis, interpretation or writing of the manuscript.
- Scragg R, Baked J, Metcalf P, Dryson E (1991) Prevalence of diabetes mellitus and impaired glucose tolerance in a New Zealand multiracial workforce. N Z Med J 104: 395-397. [Crossref]
- Sundborn G, Metcalf P, Scragg R, Schaaf D, Dyall L, et al. (2007) Ethnic differences in the prevalence of new and known diabetes mellitus, impaired glucose tolerance and impaired fasting glucose. Diabetes Heart and Health Survey (DHAH) 2002 - 2003. N Z Med J 120: U2607. [Crossref]
- Simmons D (1996) Epidemiology of diabetes and its complications in New Zealand. Diabet Med 13: 371-375. [Crossref]
- Collins J, Metcalf P (2003) Access to dialysis in New Zealand renal services.N Z Med J116: U455. [Crossref]
- Joshy G, Simmons D (2006) Epidemiology of diabetes in New Zealand: revisit to a changing landscape.N Z Med J119: U1999. [Crossref]
- Papali'i-Curtin AT, Dalziel DM (2013) Prevalence of diabetic retinopathy and maculopathy in Northland, New Zealand: 2011-2012.N Z Med J126: 20-28. [Crossref]
- Selvin E, Steffes MW, Ballantyne CM, Hoogeveen RC, Coresh J, et al. (2011) Racial difference in glycemic markers: a cross-sectional analysis of community-based data. Ann Intern Med 154: 303-309. [Crossref]
- Tsugawa Y, Mukamal KJ, Davis RB, Taylor WC, Wee CC (2012) Should the hemoglobin A1c diagnostic cutoff differ between blacks and whites? Ann Intern Med 157: 153-159. [Crossref]
- Kirk JK, D'Agostino RB Jr, Bell RA, Passmore LV, Bonds DE, et al. (2006) Disparities in HbA1c levels between African-American and non-Hispanic white adults with diabetes: a meta-analysis. Diabetes Care 29: 2130-2136. [Crossref]
- Kirk JK, Passmore LV, Bell RA, Narayan KM, D'Agostino RB Jr, et al. (2008) Disparities in A1C levels between Hispanics and non-Hispanic white adults with diabetes: a meta-analysis. Diabetes Care 31: 240-246. [Crossref]
- Herman WH, Dungan KM, Wolffenbuttel BHR, Buse JB, Fahrbach JL, et al. (2009) Racial and ethnic differences in mean plasma glucose, hemoglobin A1c, and 1,5-Anhydroglucitol in over 2000 patients with type 2 diabetes. J Clin Endocrinol Metab 94: 1689-1694. [Crossref]
- Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, et al. (2007) Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care 30: 2453-2457. [Crossref]
- Modan M, Meytes D, Rozeman P, Yosef SB, Sehayek E, et al. (1988) Significance of high HbA1c level in normal glucose tolerance. Diabetes Care 11: 422-428. [Crossref]
- Yudkin JS, Forrest RD, Jackson CA, Ryle AJ, Davies SJ, et al. (1990) Unexplained variability of glycated hemoglobin in non-diabetic subjects not related to glycemia. Diabetologia 33: 208-215. [Crossref]
- Braatvedt G, Gamble G, Kyle C (2006) Metabolic characteristics of patients with apparently normal fasting plasma glucose. N Z Med J 119: U2123. [Crossref]
- Cohen RM, Khera PK, Joiner CH, Holmes YR, Chenier TC (2002) Population variation in the steady state glucose gradient across the human erthrocyte membrane: is it a source of variation in HbA1c (Abstract). Diabetes 51: A181.
- English E, Idris I, Smith G, Dhatariya K, Kilpatrick ES, et al. (2015) The effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis: a systematic review.Diabetologia58: 1409-1421. [Crossref]
- Hempe JM, Gomez R, McCarter RJ Jr, Chalew SA (2002) High and low hemoglobin glycation phenotypes in type 1 diabetes: a challenge for interpretation of glycemic control.J Diabetes Complications16: 313-320. [Crossref]
- American Diabetes Association (2015) Classification and diagnosis of diabetes.Diabetes Care38 Suppl: S8-8S16. [Crossref]
- WHO Guidelines Approved by the Guidelines Review Committee (2011) Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. Abbreviated report of a WHO consultation. Geneva, Switzerland. [Crossref]
- Selvin E, Steffes MW, Gregg E, Brancati FL, Coresh J (2011) Performance of A1C for the classification and prediction of diabetes.Diabetes Care34: 84-89. [Crossref]
- Hempe JM, Liu S, Myers L, McCarter RJ, Buse JB, et al. (2015) The hemoglobin glycation index identifies subpopulations with harms or benefits from intensive treatment in the ACCORD trial. Diabetes Care 38: 1067-1074. [Crossref]
- Statistics New Zealand (2006) 2006 Census [Online]. [cited 2014 Oct 20.].
- Alberti KG, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation.Diabet Med15: 539-553. [Crossref]
- Viana VAG (1980) Statistical Methods for Summarizing Independent Correlational Results. J Ed Stat 5: 83-104. [Crossref]
- SAS Institute Inc (2012) SAS/STAT User's Guide. Version 9.4. SAS Institute Inc. Cary, NC.
- Eberhardt MS, Lackland DT, Wheeler F, German RR, Teutsch SM (1994) Is race related to glycemic control? An assessment of glycosylated hemoglobin in two South Carolina communities. J Clin Epidemiol 47: 1181-1189. [Crossref]
- Ziemer DC, Kolm P, Weintraub WS, Vacarino V, Rhee MK, et al. (2010) Glucose-independent, black-white difference in HbA1c levels: a cross sectional analysis of 2 studies. Ann Intern Med 152: 770-777. [Crossref]
- Kilpatrick E, Maylor P, Keevil B (1998) Biological variation of glycated hemoglobin: implications for diabetes screening and monitoring. Diabetes Care 212:261-264. [Crossref]
- Selvin E, Zhu H, Brancati FL (2009) Elevated A1C in adults without a history of diabetes in the U.S.Diabetes Care32: 828-833. [Crossref]
- Cohen RM, Ciraolo P, Palascak MB, Lindsell CJ, Khera PK, et al. (2007) Red blood cell (RBC) survival differences among hematologically normal people with diabetes (DM) make a clinically important difference in HbA1c (Abstract). Diabetes 56: A116.
- Rhee MK, Cook CB, Dunbar V, Panayioto RM, Berkowitz KJ, et al. (2005) Limited health care access impairs glycemic control in low income African Americans with type 2 diabetes. J Health Care Poor Underserved 16: 734-746. [Crossref]
- Cohen RM, Blinko SB, Snieder H, Edwards R, Lindsell CJ, et al. (2006) Evidence for independent heritability of the glycation gap (glycosylation gap) fraction of HbA1c in nondiabetic twins. Diabetes Care 29: 1739-1743. [Crossref]
- Soranzo N, Sanna S, Wheeler E, Gieger C, Radke D, et al. (2010) Common variants at 10 genomic loci influence hemoglobin A1c levels via glycemic and nonglycemic pathways. Diabetes 59: 3229-3239. [Crossref]
- Christensen DL, Witte DR, Kaduka L, Jørgensen ME, Borch-Johnsen K, et al. (2010) Moving to an A1C-based diagnosis of diabetes has a different impact on prevalence in different ethnic groups. Diabetes Care 33: 580-582. [Crossref]
- Herman WH, Cohen RM (2012) Racial and Ethnic Differences in the Relationship between HbA1c and Blood Glucose: Implications for the Diagnosis of Diabetes. J Clin Endocrinol Metab 97: 1067-1072. [Crossref]
- Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, et al. (2008) Translating the A1C assay into estimated average glucose values.Diabetes Care31: 1473-1478. [Crossref]
- Nayak AU, Nevill AM, Bassett P, Singh BM (2013) Association of glycation gap with mortality and vascular complications in diabetes.Diabetes Care36: 3247-3253. [Crossref]
- Monnier L, Lapinski H, Colette C (2003) Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients. Diabetes Care 26: 881-885. [Crossref]

