Sorafenib is an oral multikinase inhibitor that has been approved to treat advanced hepatocellular carcinoma (HCC), although it is unclear whether the intrahepatic lesion is associated with prognosis of advanced HCC patients with extrahepatic metastasis treated with sorafenib. This study aimed to assess survival risk factors and evaluate therapeutic strategies for advanced HCC patients with extrahepatic metastasis treated with sorafenib. We analyzed the clinical data and treatment outcomes for 524 consecutive advanced HCC patients treated with sorafenib. Among them, 288 (55%) patients had extrahepatic metastasis. Among the patients with extrahepatic metastasis, 212 (74%) also had intrahepatic lesion. Multivariate analyses of overall survival identified intrahepatic lesion as an independent risk factor in patients with extrahepatic metastasis. Of those, 106 (50%) were treated with sorafenib monotherapy and 106 (50%) with alternatives to sorafenib treatment. The median survival time was 6.1 months for patients with sorafenib monotherapy and 11.6 months for those administered alternative treatments (p=0.0015). Our results indicated that sorafenib treatment may have negative long-term therapeutic effects in advanced HCC patients with extrahepatic metastasis and intrahepatic lesion, and that alternative treatments should be considered for these patients after sorafenib treatment.
Hepatocellular carcinoma (HCC), one of the most prevalent types of cancer worldwide [1-4], is a major histological subtype of liver cancer, accounting for about four-fifths of total primary liver cancer cases [5,6]. The prognosis of HCC is dependent on the stage of the disease at diagnosis. However, even with treatments such as surgical resection, liver transplantation, and ablative therapies, which are only suitable for early-stage HCC patients, the majority of patients are likely to progress onto the late stages of the disease. The therapy landscape for late-stage HCC has changed with the advent of molecular-targeted therapy [7]. Sorafenib, a molecular targeted therapy for late-stage HCC, has been approved for use in Japan in May 2009 [4,8-10].
The tyrosine kinase inhibitor sorafenib induces tumor cell apoptosis. Its targets include multiple kinases, such as vascular-endothelial and platelet-derived growth factor receptors, as well as the proto-oncoprotein c-Raf and other molecules [11-14]. The Sorafenib HCC Assessment Randomized Protocol (SHARP) [15] and Asia-Pacific studies demonstrated that the effectiveness and safety of sorafenib in advanced HCC patients [16]. As the duration of survival benefit is determined by disease condition, patients undergo sorafenib treatment with the aim of maintaining stable disease (SD). In spite of the multiple clinical trials conducted to test molecular therapeutic agents other than sorafenib, no agent has been found to have an efficacy superior to that of sorafenib when it comes to treating unresectable HCC [17-20].
Only a few second-line therapies for late-stage HCC after sorafenib are available, and the selection of a treatment modality after first progression remains controversial [14]. For patients who develop progressive disease (PD) after treatment with sorafenib, the medication is often discontinued and a second-line treatment is initiated [21,22]. Recently, regorafenib is a systemic treatment that has shown to provide survival benefit in HCC patients who progressed after sorafenib treatment [23]. However, the number of patients with advanced HCC eligible for regorafenib after sorafenib failure is limited, and they are generally fewer than those who are candidates for second-line treatment [24].
Extrahepatic metastasis of HCC remains the leading cause of death from the disease [25]. Previous studies reported that these patients had poorer prognosis than those without extrahepatic metastasis [26]. To date, the prognostic factors for patients with extrahepatic metastasis treated with sorafenib remain unclear. Thus, the clinical outcome and prognosis of patients with extrahepatic metastasis treated with sorafenib require further investigation.
The controllability of intrahepatic lesions was identified as an important prognostic factor in patients with advanced HCC who had extrahepatic metastasis [27]. However, it remains unclear whether the intrahepatic lesion affects the prognosis of advanced HCC patients with extrahepatic metastasis treated with sorafenib. This study aimed to prospectively assess survival risk factors and evaluate therapeutic strategies for advanced HCC patients with extrahepatic metastasis treated with sorafenib.
Patients
The eligibility criteria for this study were similar to those of the SHARP trial [15] and were equivalent to the criteria we employed in our previous studies [4,8,10]. All enrolled patients met the following criteria: (a) Eastern Cooperative Oncology Group performance status of 0–1, (b) measurable disease using the Response Evaluation Criteria in Solid Tumors (RECIST) [28], (c) Child-Pugh class A or B liver function, (d) leukocyte count of ≥2,000/mm3, (e) platelet count of ≥50×109/L, (f) hemoglobin level of ≥8.5 g/dL, (g) serum creatinine level of <1.5 mg/dL, and (h) no ascites or encephalopathy. We enrolled 524 consecutive patients who were diagnosed with advanced HCC between May 2009 and July 2017 and who received sorafenib. HCC was either confirmed histologically or diagnosed using noninvasive criteria according to the European Association for the Study of Liver [29]. Enrolled patients were treated with sorafenib at 1 of the 17 experienced member institutions of the Kurume Liver Cancer Study Group of Japan: Asakura Medical Association Hospital, Chikugo City Hospital, Iwamoto Clinic, Kurume Central Hospital, Kurume General Hospital, Kurume University Medical Center, Kurume University School of Medicine, Kyushu Medical Center, Nagata Hospital, Ōmuta City Hospital, Saga Central Hospital, Social Insurance Tagawa Hospital, St. Mary’s Hospital, Tobata Kyoritsu Hospital, Yame General Hospital, Yanagawa Hospital, and Yokokura Hospital. The primary outcome of this study was overall survival time, which was defined as the time from initiation of sorafenib treatment until death of any cause or last follow-up. Relevant data from all patients’ clinical records, including medical history, laboratory results, radiological findings, histological results, and survival data, as well as the dosage and adverse events associated with sorafenib therapy, were prospectively collected. The study protocol was approved by the Ethics Committee of Kurume University (No. 10009) and the University Hospital Medical Information Network (UMIN) Center (no. UMIN000007427) and conformed to the guidelines of the 1975 Declaration of Helsinki. Patients were given comprehensive information on the details of the clinical study, and each provided written informed consent prior to participation.
Diagnosis
Intrahepatic lesions and vascular invasion were diagnosed using a combination of contrast-enhanced computed tomography (CT), magnetic resonance imaging (MRI), ultrasonography (US), and digital subtraction angiography. Additionally, alpha-fetoprotein (AFP), lens culinaris agglutinin-reactive fraction of AFP (AFP-L3), and des-gamma-carboxy prothrombin (DCP) serum levels were measured 1 month before treatment. Intra-abdominal metastases were detected via abdominal CT, MRI, and US, which were performed to evaluate intrahepatic lesions. Pulmonary lesions were detected on chest radiography or CT, which was routinely performed 1 month before treatment. Additional examinations, such as bone scintigraphy and brain CT or MRI, were indicated when symptoms attributable to extrahepatic metastasis appeared. These examinations were also conducted when AFP, AFP-L3, or DCP levels were elevated but were not related to the status of the intrahepatic lesions [29]. Tumor stage was determined according to the Barcelona Clinic Liver Cancer (BCLC) staging classification [30].
Sorafenib treatment
Performance status was used to determine the initial sorafenib dose, at the discretion of the chief physician. Discontinuation and dose reduction were allowed based on tolerance. Adverse events of sorafenib treatment were documented according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Treatments were discontinued if CTCAE grade 3 or higher adverse events occur, excluding those patients with platelet count of <25×109/L and leukocyte count of <1,500/mm3.
Assessment of tumor response
Imaging studies were performed 4 weeks after the initiation of sorafenib treatment and every 4–6 weeks thereafter to assess tumor response. The assessment was conducted according to the RECIST, version 1.1 [28]: complete response (CR), all measurable lesions disappeared for more than 4 weeks; partial response (PR), the sum of the diameters of the largest target lesions decreased by more than 30%, and no new lesions developed for more than 4 weeks; PD, the sum of the largest diameters increased by more than 20%, or a new lesion appeared; and SD, neither PR nor PD was observed [31]. Patients who died before their first radiographic assessment were classified as having PD. The time to radiologic progression was defined as the time from sorafenib treatment initiation until disease progression. Data of patients who died without tumor progression were censored. The disease-control rate was defined, on the basis of independent radiologic review, as the percentage of patients whose best-response RECIST rating of CR, PR, or SD was maintained for at least 1 month after the first demonstration of such a rating.
Statistical analysis
Baseline patient characteristics were analyzed using descriptive statistical methods. Survival curves were calculated using the Kaplan-Meier method. Univariate analysis of survival curves was performed using the log-rank test. A p-value of <0.05 was considered significant. The Cox proportional-hazards model was used to evaluate the interaction between baseline characteristics and the effect of sorafenib on overall survival. JMP software (SAS Institute, Inc., Cary, NC, USA), version 13, was used for all analyses.
Patient characteristics
Table 1 shows the characteristics of the enrolled patients (n=524). The mean age of the patients was 72 years. Of the total study population, 414 (79%) were men and 110 (21%) were women. The main causes of HCC were chronic hepatitis C (n=309; 59%) and hepatitis B (n=100; 19%) viral infections. Of the patients enrolled in our study, 241 (46%) had a Child-Pugh score of 5, 173 (33%) had a Child-Pugh score of 6, 414 (79%) had Child-Pugh class A, and 110 (21%) had class B liver cirrhosis. About 126 (24%) patients had macrovascular invasion, while 398 (76%) had no macrovascular invasion. Based on the BCLC staging system, 168 (32%) patients had stage B, and 356 (68%) had stage C.
Table 1. Patient characteristics (n=524). HBV=hepatitis B virus, HCV=hepatitis C virus, BCLC= Barcelona Clinic Liver Cancer, AFP=alpha-fetoprotein, DCP=des-gamma-carboxy prothrombin. Results are presented as n (%) or median [range]
Variable |
Value |
Age, years [range] |
72 [33–94] |
Sex, n (%) |
|
Male |
414 (79) |
Female |
110 (21) |
Etiology, n (%) |
|
HBV |
100 (19) |
HCV |
309 (59) |
Negative for hepatitis virus |
115 (22) |
Child-Pugh class, n (%) |
|
A |
414 (79) |
B |
110 (21) |
Macrovascular invasion, n (%) |
|
Present |
126 (24) |
Absent |
398 (76) |
Tumor stage, n (%) |
|
BCLC - B |
168 (32) |
BCLC - C |
356 (68) |
Daily initial sorafenib dose, n (%) |
|
400 mg |
372 (71) |
800 mg |
152 (29) |
Extrahepatic metastasis, n (%) |
288 (55) |
Lung |
168 (32) |
Lymph node |
68 (13) |
Bone |
52 (10) |
Peritoneum |
26 (5) |
Adrenal gland |
16 (3) |
Albumin, median in g/L [range] |
3.60 [2.10–4.80] |
Total bilirubin, median in mg/dL [range] |
0.80 [0.15–3.70] |
Prothrombin time, median in % [range] |
82.5 [10.8–136.0] |
AFP, median in ng/mL [range] |
116 [1–987600] |
AFP L3, median in % [range] |
23.8 [0.0–99.6] |
DCP, median in mAU/mL [range] |
667 [2–1590000] |
Treatment compliance
The daily initial sorafenib dose was 400 mg for 372 (71%) patients and 800 mg for 152 (29%) patients. The daily average sorafenib dose was over 400 mg for 267 (51%) patients and less than 400 mg for 257 (49%) patients. At the end of the follow-up period, 477 (91%) patients had discontinued treatment due to various reasons, including adverse reactions in 257 (49%) patients, radiologic and symptomatic progression in 147 (28%), and worsening of performance status in 47 (9%); moreover, 26 (5%) patients requested to discontinue their treatment.
Extrahepatic metastasis
Of the treated patients, 288 (55%) had extrahepatic metastasis, while 236 (45%) had no extrahepatic metastasis. The most frequent sites of extrahepatic metastases were the lungs (n=168; 32%), followed by lymph nodes (n=68; 13%), bones (n=51; 10%), peritoneum (n=26; 5%), and adrenal glands (n=16; 3%).
Overall response and efficacy
Of the enrolled patients, 424 (81%) were treated with sorafenib for a month. The median duration of sorafenib treatment was 3.7 months (range: 0.1–60.8 months), and the median follow-up period was 9.2 months (range: 0.4–92.7 months). About 419 (80%) patients died during the observation period, while 105 (20%) survived throughout the follow-up period. The cumulative survival curves for all patients in Figures 1 and 2 yielded a median survival time (MST) of 10.5 months (range: 0.4–92.7 months; Figure 1), a 1-year survival rate of 46%, and a median progression-free survival (PFS) time of 3.9 months (range: 0.1–75.0 months; Figure 2). Table 2 shows the results of the first radiologic assessment according to the RECIST: 31 (6%) patients showed PR, 199 (38%) had SD, and 257 (49%) experienced PD. However, the results of the follow-up radiologic evaluation conducted in 31 (6%) patients were unavailable. Thus, the disease control rate was 44%.
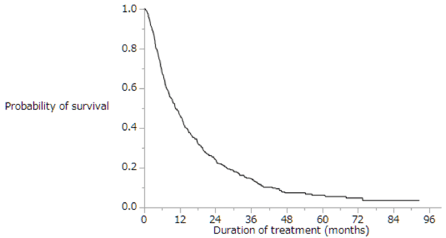
Figure 1. Kaplan-Meier analysis of overall survival rates of the enrolled patients. Median survival time was 10.5 months, and the 1-year survival rate was 46%
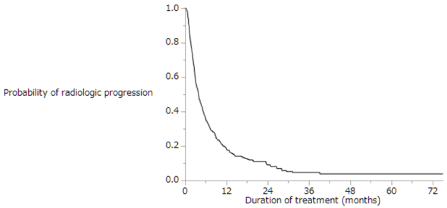
Figure 2. Kaplan-Meier analysis of radiologic progression-free survival of the enrolled patients. Median survival time was 3.9 months
Table 2. Therapeutic effects in all patients (n=524). PR=partial response, SD=stable disease, PD=progressive disease
Therapeutic effects |
n (%) |
PR |
31 (6) |
SD |
199 (38) |
PD |
257 (49) |
Not evaluable |
31 (6) |
Factors correlated with survival outcome in patients with extrahepatic metastasis
Figure 3 shows Kaplan-Meier analysis of overall survival in patients with (dotted line; n=288) and without (solid line; n=236) extrahepatic metastasis. Median survival time was 11.9 months vs. 9.6 months, respectively (p=0.8259). The overall survival did not differ significantly between patients with extrahepatic metastasis and those without extrahepatic metastasis. Table 3 shows univariate and multivariate analyses of overall survival in patients with extrahepatic metastasis (n=288). Univariate analyses of overall survival in patients with extrahepatic metastasis identified eight baseline patient characteristics as prognostic indicators: Child-Pugh class, intrahepatic lesion, macrovascular invasion, duration of sorafenib treatment, daily initial sorafenib dose, serum AFP level at baseline, serum DCP level at baseline, and therapeutic effect. Multivariate analyses of overall survival in patients with extrahepatic metastasis identified three baseline patient characteristics as prognostic indicators: intrahepatic lesion, duration of sorafenib treatment, and serum DCP level at baseline. Univariate and multivariate analyses showed that presence of intrahepatic lesion was a significant risk factor in patients with extrahepatic metastasis.
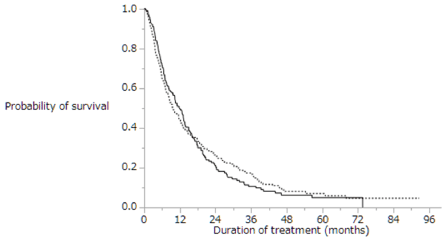
Figure 3. Kaplan-Meier analysis of the overall survival rates of patients with (dotted line; n=288) and without (solid line; n=236) extrahepatic metastasis. Median survival time was 11.9 months vs. 9.6 months, respectively (p=0.8259)
Table 3. Univariate and multivariate analyses of the overall survival of patients with extrahepatic metastasis (n=288). HR=hazard ratio, CI=confidence interval, AFP=alpha-fetoprotein, DCP=des-gamma-carboxy prothrombin, PD=progressive disease. Results are presented as n or mean ± standard deviation
Variable |
Univariate analysis |
Multivariate analysis |
HR (95% CI) |
p-value |
HR (95% CI) |
p-value |
Age (≥72 years) |
1.026 (0.771–1.364) |
0.8555 |
1.022 (0.684–1.525) |
0.9150 |
Sex (male) |
0.739 (0.532–1.047) |
0.0883 |
0.775 (0.508–1.215) |
0.2598 |
Child-Pugh class (B) |
1.944 (1.389–2.677) |
0.0002 |
1.347 (0.834–2.107) |
0.2158 |
Intrahepatic lesion (present) |
1.899 (1.358–2.713) |
0.0001 |
1.792 (1.158–2.823) |
0.0085 |
Macrovascular invasion (present) |
1.408 (1.014–1.926) |
0.0412 |
0.861 (0.501–1.443) |
0.5778 |
Duration of sorafenib treatment (≥3.7 months) |
0.292 (0.218–0.392) |
<0.0001 |
0.273 (0.183–0.407) |
<0.0001 |
Daily initial sorafenib dose (800 mg) |
0.659 (0.478–0.896) |
0.0076 |
0.776 (0.511–1.162) |
0.2214 |
Daily average sorafenib dose (≥400 mg) |
1.032 (0.777–1.371) |
0.8249 |
1.080 (0.744–1.566) |
0.6828 |
AFP (≥116 ng/ml) |
1.378 (1.036–1.834) |
0.0275 |
1.164 (0.770–1.764) |
0.4702 |
AFP-L3 (≥23.8 %) |
1.234 (0.888–1.715) |
0.2081 |
0.825 (0.542–1.249) |
0.3662 |
DCP (≥667 mAU/ml) |
1.670 (1.252–2.232) |
0.0005 |
1.725 (1.169–2.556) |
0.0060 |
Therapeutic effect (PD) |
1.782 (1.322–2.417) |
0.0001 |
1.108 (0.762–1.613) |
0.5879 |
Analysis of patients with extrahepatic metastasis and intrahepatic lesion
Figure 4 shows the Kaplan-Meier analysis of overall survival in patients with extrahepatic metastasis with (dotted line; n=212) and without (solid line; n=76) intrahepatic lesion. Median survival time was 8.6 months vs. 23.3 months, respectively (p=0.0002). Patients with extrahepatic metastasis with intrahepatic lesion had shorter overall survival than those without intrahepatic lesion. Table 4 shows univariate and multivariate analyses of overall survival in patients with extrahepatic metastasis and intrahepatic lesion (n=212). Univariate analyses of overall survival in patients with extrahepatic metastasis and intrahepatic lesion identified six baseline patient characteristics as prognostic indicators: Child-Pugh class, alternative treatments, duration of sorafenib treatment, daily initial sorafenib dose, serum DCP level at baseline, and therapeutic effect. Multivariate analyses of overall survival in patients with extrahepatic metastasis identified two baseline patient characteristics as prognostic indicators: alternative treatments and duration of sorafenib treatment. Univariate and multivariate analyses showed that the patients treated with alternatives to sorafenib survived longer than those treated with sorafenib monotherapy. Table 5 shows alternative treatments for patients with extrahepatic metastasis and intrahepatic lesion after sorafenib treatment (n=212): 51 (24%) patients underwent transcatheter arterial chemoembolization (TACE), 47 (22%) were treated with hepatic arterial infusion chemotherapy (HAIC), 28 (13%) were treated with radiation therapy, 21 (10%) were treated with systemic chemotherapy except for sorafenib, and 4 (2%) underwent hepatic resection. Some patients in the groups received multiple treatments. Figure 5 shows Kaplan-Meier analysis of overall survival in patients with extrahepatic metastasis and intrahepatic lesion treated with sorafenib monotherapy (solid line; n=106) and with alternatives to sorafenib treatment (dotted line, n=106). Median survival time was 6.1 months vs. 11.6 months, respectively (p=0.0015). Patients with extrahepatic metastasis and intrahepatic lesion treated with alternatives to sorafenib treatment survived significantly longer than those treated with sorafenib monotherapy.
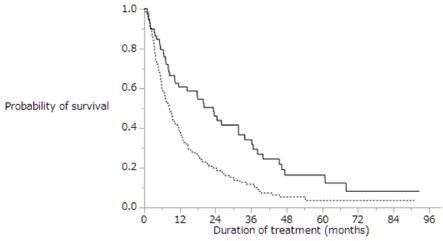
Figure 4. Kaplan-Meier analysis of the overall survival rates of patients with extrahepatic metastasis with (dotted line; n=212) and without (solid line; n=76) intrahepatic lesion. Median survival time was 8.6 months vs. 23.3 months, respectively (p=0.0002)
Table 4. Univariate and multivariate analyses of the overall survival of patients with extrahepatic metastasis and intrahepatic lesion (n=212). CR=complete response, PR=partial response, SD=stable disease, PD=progressive disease, CI=confidence interval, AFP=alpha-fetoprotein, DCP=des-gamma-carboxy prothrombin, NFP=new 5-fluorouracil and cisplatin therapy. Results are presented as n or mean ± standard deviation
Variable |
Univariate analysis |
Multivariate analysis |
HR (95% CI) |
p-value |
HR (95% CI) |
p-value |
Age (≥72 years) |
0.982 (0.711–1.356) |
0.9147 |
0.855 (0.531–1.386) |
0.5243 |
Sex (male) |
0.770 (0.538–1.128) |
0.1759 |
0.737 (0.461–1.213) |
0.2247 |
Child-Pugh class (B) |
1.771 (1.221–2.523) |
0.0030 |
1.576 (0.904–2.675) |
0.1065 |
Macrovascular invasion (present) |
1.247 (0.880–1.744) |
0.2090 |
1.013 (0.543–1.835) |
0.9662 |
Alternative treatments (present) |
0.593 (0.428–0.822) |
0.0018 |
0.259 (0.150–0.441) |
<0.0001 |
Duration of sorafenib treatment (≥3.7 months) |
0.258 (0.182–0.362) |
<0.0001 |
0.123 (0.067–0.220) |
<0.0001 |
Daily initial sorafenib dose (800 mg) |
0.664 (0.460–0.942) |
0.0214 |
0.789 (0.464–1.319) |
0.3705 |
Daily average sorafenib dose (≥400 mg) |
1.011 (0.732–1.398) |
0.9451 |
0.982 (0.615–1.569) |
0.9399 |
AFP (≥116 ng/ml) |
1.352 (0.977–1.881) |
0.0686 |
1.337 (0.813–2.201) |
0.2511 |
AFP-L3 (≥23.8 %) |
1.188 (0.812–1.747) |
0.3736 |
0.690 (0.406–1.160) |
0.1627 |
DCP (≥667 mAU/ml) |
1.623 (1.169–2.264) |
0.0037 |
1.302 (0.787–2.174) |
0.3045 |
Therapeutic effect (PD) |
1.757 (1.252–2.486) |
0.0011 |
0.877 (0.538–1.427) |
0.5969 |
Table 5. Alternative treatments for patients with extrahepatic metastasis and intrahepatic lesion after sorafenib treatment (n=212). TACE=transcatheter arterial chemoembolization, HAIC=hepatic arterial infusion chemotherapy. Some patients in both groups received multiple treatments
Alternative treatments |
n (%) |
TACE |
51 (24) |
HAIC |
47 (22) |
Radiation therapy |
28 (13) |
Systemic chemotherapy except for sorafenib |
21 (10) |
Hepatic resection |
4 (2) |
Sorafenib is an oral multikinase inhibitor that has been used for molecularly targeted therapy of late-stage HCC patients [4]. Sorafenib treatment is well tolerated and has improved patient survival in two phase III clinical trials that were randomized and placebo controlled [15,16]. In our current study, we assessed the prognostic indicators in advanced HCC patients with extrahepatic metastasis and evaluated alternative treatments in patients with extrahepatic metastasis and intrahepatic lesion. The MST (10.5 months) of sorafenib-treated patients was longer in our study (Figure 1) than in the Asia-Pacific study (6.5 months) [16] while being comparable to the MST observed for SHARP patients (10.7 months) [15].
The prognosis of HCC patients with extrahepatic metastases was unsatisfactory [32,33]. In our current study, we assessed the prognosis of 524 consecutive HCC patients treated with sorafenib with or without extrahepatic metastases. We found that the most frequent metastatic sites were the lungs, followed by the lymph nodes and bone (Table 1). The overall survival time in patients treated with sorafenib did not differ significantly between patients with extrahepatic metastasis and those without extrahepatic metastasis (Figure 3). Thus, the presence of extrahepatic metastasis might not affect the prognosis of advanced HCC patients treated with sorafenib, as reported in our previous study [4]. Therefore, these patients should be considered for sorafenib treatment [4]. On the contrary, extrahepatic metastasis of HCC remains the leading cause of death [25]. Previous studies reported that these patients had poorer prognosis than those without extrahepatic metastasis [26]. To date, the prognostic factors for patients with extrahepatic metastasis treated with sorafenib remain unclear. Thus, the clinical outcome and prognosis of patients with extrahepatic metastasis treated with sorafenib require further investigation.
Using exploratory univariate analysis, we identified eight prognostic indicators of survival in advanced HCC patients with extrahepatic metastasis treated with sorafenib, including the presence of intrahepatic lesion (Table 3). We demonstrated that the presence of intrahepatic lesion was an independent risk factor that negatively affected survival in advanced HCC patients with extrahepatic metastasis treated with sorafenib (Figure 4). The progression of an intrahepatic lesion was the major cause of death among these patients; thus, we need to consider alternative treatments such as surgical resection, ablative therapies, TACE, or HAIC based on the status of the intrahepatic lesion. Our previous study showed that HAIC was a significantly positive prognostic treatment for patients with advanced HCC and macrovascular invasion. Especially when macrovascular invasion is present, HAIC is considered to be a suitable alternative treatment.
Using exploratory univariate analysis, we identified six prognostic indicators of survival in advanced HCC patients with extrahepatic metastasis and intrahepatic lesion treated with sorafenib, including the alternative treatments (Table 4). We demonstrated that the alternative treatments for intrahepatic lesion was an independent risk factor that positively affected the survival of advanced HCC patients with extrahepatic metastasis and intrahepatic lesion treated with sorafenib (Figure 5). While previous clinical trials showed that sorafenib can prolong the survival of patients with advanced HCC [15,16], these studies made no recommendations as to whether sorafenib should be continued when a patient develops PD [14]. In our previous study, patients treated with alternatives to sorafenib had longer overall survival than those treated with sorafenib monotherapy [10]. In our current study, advanced HCC patients with extrahepatic metastasis and intrahepatic lesion treated with alternatives to sorafenib also survived longer than those treated with sorafenib monotherapy. Therefore, sorafenib should be discontinued and alternative treatments should be provided in advanced HCC patients with PD, regardless of the presence or absence of extrahepatic metastasis or intrahepatic lesion.
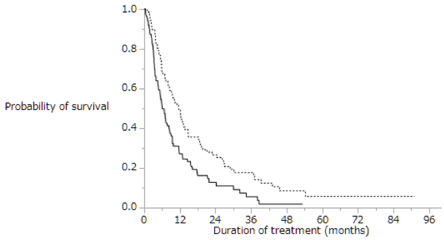
Figure 5. Kaplan-Meier analysis of the overall survival of patients with extrahepatic metastasis and intrahepatic lesion treated with sorafenib monotherapy (solid line; n=106) and with alternatives to sorafenib treatment (dotted line, n=106). Median survival time was 6.1 months vs. 11.6 months, respectively (p=0.0015)
Currently, there are no effective second-line treatments for patients with HCC who developed PD after treatment with sorafenib [22]. To improve the prognosis of these patients, clinicians must be able to predict tumor response to sorafenib treatment [20]. It is indicated that regorafenib is beneficial in patients with advanced HCC who progressed after sorafenib therapy [32-34]. However, it remains unknown which alternative treatment is better. Future trials should explore combinations of regorafenib with other systemic agents and third-line treatments for patients who fail or who do not tolerate sorafenib and regorafenib [23].
Our study has several limitations. First, the alternative treatments in patients who developed PD after sorafenib treatment were selected at the discretion of the chief physician and were not randomized. This resulted in a selection bias for patients treated with sorafenib monotherapy and those administered alternative treatments. Second, some patients in the groups other than those treated with sorafenib monotherapy received multiple treatments. Lastly, the size of the study cohort was relatively small. To confirm the superiority of alternative treatments (including the aforementioned combinatorial drugs) for abolishing sorafenib resistance in patients who develop PD after sorafenib treatment, prospective randomized studies with a larger number of subjects are required.
In conclusion, our results showed that intrahepatic lesion was a significantly negative prognostic indicator for advanced HCC patients with extrahepatic metastasis. Furthermore, in advanced HCC patients with extrahepatic metastasis and intrahepatic lesion, alternative treatment for intrahepatic lesion was a significantly positive prognostic indicator for patients who developed PD after treatment with sorafenib. Therefore, alternative treatments should be considered for patients with extrahepatic metastasis and intrahepatic lesion who developed PD after sorafenib treatment.
The authors thank the staff of the Kurume Liver Cancer Study Group of Japan for their valuable support and Editage (www.editage.jp) for providing quality English language editing services.
- El-Serag HB, Mason AC (1999) Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 340: 745-750.
- Parkin DM, Bray F, Ferlay J (2005) Global cancer statistics, 2002. Cancer Journal for Clinicians 55: 74-108.
- Sherman M (2005) Hepatocellular carcinoma: epidemiology, risk factors, and screening, Seminars in Liver Disease 25: 143-154.
- Nakano M, Tanaka M, Kuromatsu R, Nagamatsu H, Tajiri N, et al. (2015) Torimura, Sorafenib for the treatment of advanced hepatocellular carcinoma with extrahepatic metastasis: a prospective multicenter cohort study. Cancer Medicine 4: 1836-1843.
- Perz JF, Armstrong GL, Farrington FA, Hutin YJ, Bell BP, et al. (2006) The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. Journal of Hepatology 45: 529-538.
- Zhang P, Yang Y, Wen F, He X, Tang R, et a. (2015) Cost-effectiveness of sorafenib as a first-line treatment for advanced hepatocellular carcinoma. European Journal of Gastroenterology & Hepatology 27: 853-859.
- Yau T, Chan P, Ng KK, Chok SH, Cheung TT, Fan ST (2009) Phase 2 open-label study of single-agent sorafenib in treating advanced hepatocellular carcinoma in a hepatitis B-endemic Asian population: presence of lung metastasis predicts poor response. Cancer 115: 428-436.
- Nakano M, Tanaka M, Kuromatsu R, Nagamatsu H, Sakata K, et al. (2013) Efficacy, safety, and survival factors for sorafenib treatment in Japanese patients with advanced hepatocellular carcinoma. Oncology 84: 108-114.
- Furuse J, Ishii H, Nakachi K, Suzuki E, Shimizu S, Nakajima K, et al. (2008) Phase I study of sorafenib in Japanese patients with hepatocellular carcinoma. Cancer Science 99: 159-165.
- Nakano M, Tanaka M, Kuromatsu R, Nagamatsu H, Satani M, et al. (2016) Alternative treatments in advanced hepatocellular carcinoma patients with progressive disease after sorafenib treatment: a prospective multicenter cohort study. Oncotarget 7: 64400-64409.
- Amantini C, Morelli MB, Santoni M, Soriani A, Cardinali C, et al. (2015) Sorafenib induces cathepsin B-mediated apoptosis of bladder cancer cells by regulating the Akt/PTEN pathway. The Akt inhibitor, perifosine, enhances the sorafenib-induced cytotoxicity against bladder cancer cells. Oncoscience 2: 395-409.
- Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, et al. (2008) Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signalling. Molecular Cancer Therapeutics 7: 3129-3140.
- Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, et al. (2004) Trail, BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Research 64: 7099-7109.
- Miyahara K, Nouso K, Morimoto Y, Takeuchi Y, Hagihara H, et al. (2014) Efficacy of sorafenib beyond first progression in patients with metastatic hepatocellular carcinoma. Hepatology Research 44: 296-301.
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, et al. (2008) Sorafenib in advanced hepatocellular carcinoma. The New England Journal of Medicine 359: 378-390.
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. The Lancet Oncology 10: 25-34.
- Villanueva, Llovet JM (2011) Targeted therapies for hepatocellular carcinoma. Gastroenterology 140: 1410-1426.
- Pazo-Cid RA, Lanzuela M, Esquerdo G, Perez-Gracia JL, Anton A, et al. (2012) Novel antiangiogenic therapies against advanced hepatocellular carcinoma (HCC), Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico 14: 564-574.
- Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, et al. (2013) Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. Journal of Clinical Oncology 31: 3517-3524.
- Takeda H, Nishikawa H, Osaki Y, Tsuchiya K, Joko K, et al. (2015) Clinical features associated with radiological response to sorafenib in unresectable hepatocellular carcinoma: a large multicenter study in Japan. Liver International 35: 1581-1589.
- Finn RS (2013) Emerging targeted strategies in advanced hepatocellular carcinoma. Seminars in Liver Disease 33: S11-19.
- Lee IC, Chen YT, Chao Y, Huo TI, Li LP (2015) Determinants of survival after sorafenib failure in patients with BCLC-C hepatocellular carcinoma in real-world practice. Medicine 94: e688.
- Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. (2017) Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London, England) 389: 56-66.
- Ogasawara S, Chiba T, Ooka Y, Suzuki E, Maeda T, et al. (2017) Characteristics of patients with sorafenib-treated advanced hepatocellular carcinoma eligible for second-line treatment. Investigational New Drugs.
- Aino H, Sumie S, Niizeki T, Kuromatsu R, Tajiri N, et al. (2014) Clinical characteristics and prognostic factors for advanced hepatocellular carcinoma with extrahepatic metastasis. Molecular and Clinical Oncology 2: 393-398.
- Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, et al. (2007) Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World Journal of Gastroenterology 13: 414-420.
- Uchino K, Tateishi R, Shiina S, Kanda M, Masuzaki R, et al. (2011) Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer 117: 4475-4483.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, et al. (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45: 228-247.
- Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, et al. (2001) Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. Journal of Hepatology 35: 421-430.
- Forner ME, Reig CR de Lope, Bruix J (2010) Current strategy for staging and treatment: the BCLC update and future prospects. Seminars in Liver Disease 30: 61-74.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205-216.
- Natsuizaka M, Omura T, Akaike T, Kuwata Y, Yamazaki K, Sato T, et al. (2005) Clinical features of hepatocellular carcinoma with extrahepatic metastases. Journal of Gastroenterology and Hepatology 20: 1781-1787.
- Ishii H, Furuse J, Kinoshita T, Konishi M, Nakagohri T, et al. (2004) Extrahepatic spread from hepatocellular carcinoma: who are candidates for aggressive anti-cancer treatment? Japanese Journal of Clinical Oncology 34: 733-739.
- Kim K, Jha R, Prins PA, Wang H, Chacha, et al. (2017) Regorafenib in advanced hepatocellular carcinoma (HCC): considerations for treatment. Cancer Chemotherapy and Pharmacology.





