Objectives: The aim of this study was to compare the effects of platelet-rich fibrin (PRF) and low-level laser therapy (LLLT) on new bone formation in rats which underwent tooth extraction.
Materials and methods: 72 female rats were divided into 4 different groups, three groups were considered as experimental and the other as control following the extraction of their upper right central incisor. The animals in Group 1 underwent LLLT and irradiated with a dose of 12-20 J/cm2 in total in every 3 days. In Group 2, PRF was applied to the extraction sockets of the rats and was fixed with 6/0 suture. Rats in Group 3 received a combination of PRF and LLLT. Then, rats were sacrificed on the 7th, 10th and 14th day, and their upper right maxilla were removed as specimens. For histopathological evaluation, the specimens were stained with Hematoxylin-Eosin and analysed histologically and histomorphometrically under a light microscope.
Results: The amount of newly formed bone in Group 3 on the 7th, 10th and 14th days was found to be higher than the other groups. This difference in Group 3 was found to be statistically significantly higher on the 7th, 10th and 14th days compared with the control group (p<0.05). The amount of newly formed bone in Group 3 on the 10th day was found to be statistically significantly higher than Group 2 (p<0.05).
Conclusion: Based on these results, it was concluded that combined use of PRF and LLLT increases new bone formation after tooth extraction.
platelet-rich fibrin, low-level laser therapy, tooth exraction, rat
Various treatment applications have been tried to accelerate bone recovery period and to achieve the desired function faster following surgical procedures. In addition to these, the patients’ requests for implant application in the early period after surgical procedures such as tooth extraction has influenced the researchers’ quest for alternative practices. One of the most commonly used methods of physical therapy is stimulation performed using low-level laser systems. Application of low-level laser at appropriate doses has a therapeutic effect on the biostimulation of the bone tissue and bone repair [1].
Low-level laser therapy (LLLT) has been considered to be effective in controlling pain and inflammation, increasing new tissue production and wound healing. Absorbtion of the laser beam by the tissue induces biochemical pathways, leads to the activation of mitochondrial respiratory chain and essentially increases the production of ATP (adenosine triphosphate), NO (nitric oxide), and a trace amount of ROS (reactive oxygen species). As a result, LLLT accelerates cellular activities and affects the healing process at the tissue level [2,3].
Bone grafts have been widely used for the repair of bone defects and to accelerate bone healing. Studies are under way in order to overcome problems that can occur when harvesting grafts, such as donor-site morbidity, blood loss and harvesting limited amount of graft. In today’s operative dentistry, platelet-rich fibrin (PRF) is also being used in addition to various graft materials, or in combination with graft materials [4]. In PRF matrix, there are various growth factors which have important roles in wound healing, as well as cytokines that are released by cytokines and play a role in inflammation control. Cytokines in the matrix have been reported to be long-acting, due to their slow release during remodelling stage. PRF is considered to be a strong stimulator of bone formation and regeneration by increasing the proliferation of osteogenic cells. PRF helps to reduce bleeding and accelerate soft and hard tissue healing. It has many advantages such as accelerating vascularization due to growth factors, not causing allergic reactions when harvested autologously, easy preparation in a short time, absence of disease transfer risk, controlling inflammation via leukocytes and the cytokines they release, and suppression of infection [4,5].
When structural and mechanical restoration of bone tissue losses, which is one of the most important problems of modern dentistry, a good regeneration is achieved. Applications performed for this purpose are difficult due to weak blood supply, absence of mechanical stability and more active proliferation of the other tissues. In the recent years, laser biostimulation is performed in combination with regenerative methods to stimulate healing in bone defects [6].
In our study, the aim was to investigate the effects of platelet-rich fibrin and low-level laser therapy on new bone formation in subjects which underwent tooth extraction.
Experimental model
After obtaining the ethics committee approval for the study (GATA 15/47, 10.04.2015), 72 healthy, 12-week old, female wistar rats weighing between 200-300 gr were included in the study. Animals were assigned to 4 different groups, one of which was the control group. Of these animals, 12 were lost to anesthesia and tooth extraction. Rats in Group 1 underwent low-level laser therapy (LLLT) and irradiated with a dose of 12-20 J/cm2 in total in every 3 days until the day of their sacrifice. In Group 2, platelet-rich fibrin (PRF) was applied to the extraction sockets of the rats and was fixed with 6/0 suture. Rats in Group 3 received a combination of PRF and LLLT. Then, rats in each group were divided into three subgroups to be sacrificed on the 7th, 10th and 14th day.
Tooth extraction
Tooth extraction was performed immediately in the control group and Group 1, and after their sacrifice in Groups 2 and 3. Subjects in the control group were used as donors to obtain PRF. For tooth extraction, general anesthesia was induced in all rats using ketamine HCl (Alfamin 10%) 90 mg/kg I.M. and xylazine HCl (Alfazyne 2%) 10 mg/kg I.M. injection. Local anesthesia was induced using 0.1 cc articaine HCl (Fullcain Ampulla, Onfarma). The tip of a 15-blade scalpel was wedged between the upper incisors for the elevation of the tooth, and luxation was performed with the help of a bayonet, and the tooth was extracted (Figure 1). Bleeding was controlled using a small sterile tampon gauze in subjects whose tooth extraction was complete.
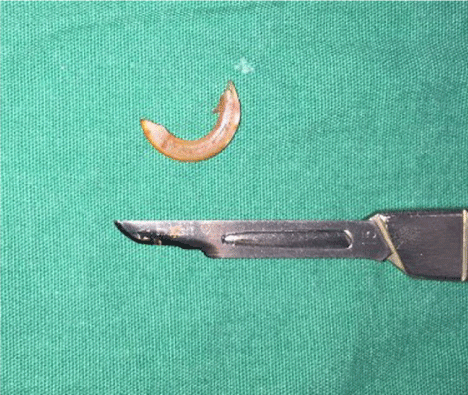
Figure 1. Image of the upper anterior incisor after extraction
Laser application
Subjects in the experiment groups were exposed to 100 mW laser (940±10 nm InGaAsP diode laser, Biolase Epic™ 10, USA) in continuous wave emission mode for 5 seconds, to ensure an irradiation area of 0.1256 cm2 in the extraction socket from a 5 mm distance. Subjects were exposed to a daily dose of approximately 4 J/cm2 (0.1 W x 5 sec / 0.1256 cm2 = 3.981 J/cm2) intraoral laser every three days (day 0, 3, 6, 9, 12) after extraction (including extraction day), on the same time. The total applied dose was 12-20 J/cm2. Laser application was performed in the same session with the tooth extraction when the subjects were under general anesthesia, and the subsequent laser applications were performed with an assistant, by immobilizing the subjects.
Application of platelet-rich fibrin
A total of 4 ml blood was collected intracardiacally from the subjects in the control group under general anesthesia, using a 22G 5ml injector on the days of sacrifice. The collected blood was transferred into glass-coated 9 ml plastic tubes without any blood coagulants and centrifuged at 3000 rpm for 12 minutes using Herme Z 326 (Hermle Labortechnik GmbH, Wehingen, Germany). After centrifugation, the layer containing red blood cells was observed at the bottom of the tube, PRF clot was observed in the middle of the tube, and acellular plasma was found at the surface. Using a cotton plier, fibrin clot was taken out of the tube and red blood cells adhering to the lower part of the fibrin were removed with scissors.
Termination of the study and collection of maxillary bone-blocks
At the end of the study, general anesthesia was induced in rats grouped according to their day of sacrifice using ketamine HCl (Alfamin 10%) 90 mg/kg I.M. and xylazine HCl (Alfazyne 2%) 10 mg/kg I.M. injection. Subjects were sacrificed via intracardiac injection of high-dose ketamine. After euthanasia, in order to examine extraction sockets, maxillary bones exposed following the dissection of cutaneous and subcutaneous tissues of the skull were resected using diamond disc along the separated zygomatic arch. The collected bone-blocks were stored in biopsy containers filled with 10% formaldehyde solution. Then, bone specimens were examined histologically to evaluate bone healing within the extraction socket.
Histological evaluation
Fixation of the specimens obtained after the sacrifice was performed using 10% buffered formalin (neutral phosphate-buffered formalin) for 24-72 hours, followed by their decalcification in 10% formic acid. 10% formic acid solutions were changed in every two days and decalcification was completed in a week, and then the specimens were washed overnight under running water and embedded into paraffin blocks following routine tissue processing procedure. For the routine hematoxyline-eosin staining of the tissues, sagittal sections at an approximately 4-5 mm thickness were obtained and placed on adhesive slides (Surgipath, X-tra Adhesive Microslides, Illinois, USA).
Sections were deparaffinized inside a 70oC incubator for an hour, followed by two 30-minute incubations in xylol and alcohol, respectively, washed under tap water, and stained. Specimens stained with routine hemotxyline-eosine (HE) staining procedure for histopathological evaluation were histologically and histomorphometrically analysed under Leica DM 4000 B (Leica Microsystems GmbH. Wetzlar, Germany) light microscope.
Extraction sockets were evaluated in terms of the amount of newly formed bone and the number of inflammatory cells observed during healing. The amount of new bone was calculated using semi-quantitative method, by measuring the amount of bone formed within the entire socket (µm2) under x400 magnification with 100-unit ocular grid [7,8].
Statistical evaluation
Data obtained in this study was analyzed using IBM SPSS Statistics Version 20 (Statistical Package for the Social Sciences, Chicago, IL) software package. Normal distribution of the variables were analyzed using Shapiro Wilk’s test due to unit numbers. Friedman’s Two Way ANOVA was used in the analysis of more than two dependent samples since they were non-normally distributed; and if significant differences were found, multiple comparison tests were used and variables that differ from each other were detected. When analyzing inter-group differences, if the variables are non-normally distributed, Kruskal Wallis-H test was used. If significant differences were detected with Kruskal Wallis H test, Post-Hoc Multiple Comparison test was used to determine the groups that differ from each other. When interpreting the results, a significance level of 0.05 was used; and if p<0.05, it was concluded that there is a significant relationship, and if p>0.05, it was concluded that the relationship is not significant.
Histological results
Control group
On the 7th day, approximately 5-10% bone matrix formation was observed at the base of the extraction socket, filling 1/3 of the socket, in all specimens. On the 10th day, approximately 20% osteoid formation was observed at the base of the socket, filling 1/3 of the socket. On the 14th day, 40% new bone trabeculae at the base of the extraction socket was observed (Figure 2).
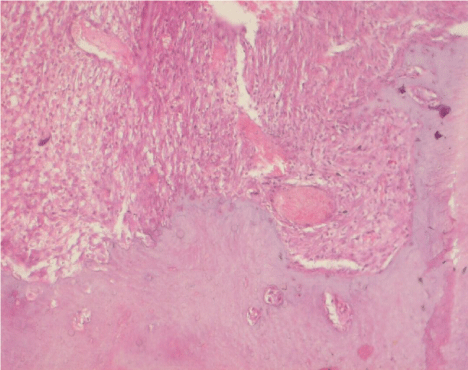
Figure 2. Histologic image of the extraction sockets at day 14 after tooth extraction in the control group (H&E, ×20)
Test group 1 (LLLT)
On the 7th day, granulation tissue at the base of the socket, filling 1/3 of the socket, was observed and 10-20% bone trabeculae at the base of the socket was observed. On the 10th day, approximately 30% new bone trabeculae was observed in the granulation tissue. On the 14th day, in all specimens, 40% new bone trabeculae at the base of the socket, filling 1/3 of the socket, was observed (Figure 3).
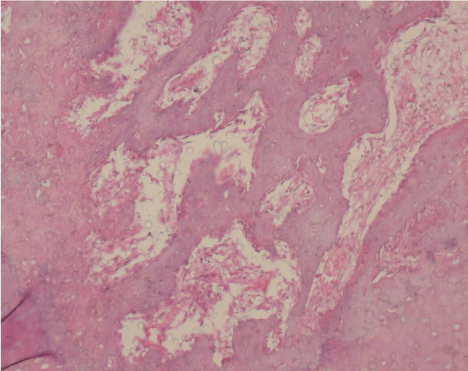
Figure 3. Histologic image of the extraction sockets at day 14 in the LLLT group (H&E, ×20)
Test group 2 (PRF)
On the 7th day, 5-10% bone matrix formation was detected at the base of the extraction socket, filling 1/3 of the socket. On the 10th day, 20-30% new bone trabeculae was observed at the base of the extraction socket, filling 1/3 of the socket. On the 14th day, approximately 40% new bone trabeculae was detected at the base of the extraction socket (Figure 4).
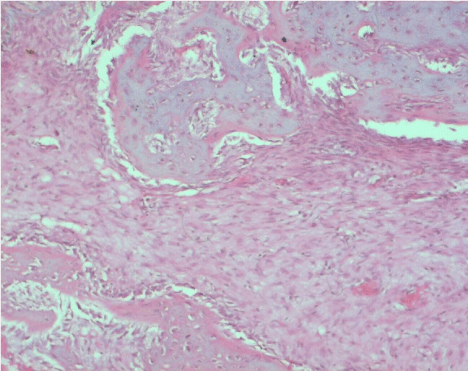
Figure 4. Histologic image of the extraction sockets at day 14 in the PRF group (H&E, ×20)
Test group 3 (LLLT+PRF)
On the 7th day, in all specimens, approximately 30% new bone trabeculae at the base of the socket, filling 1/3 of the extraction socket, was observed. On the 10th day, approximately 30-40% new bone trabeculae was detected in the granulation tissue. On the 14th day, approximately 50-60% of new bone trabeculae was detected in the specimens of this group (Figure 5).
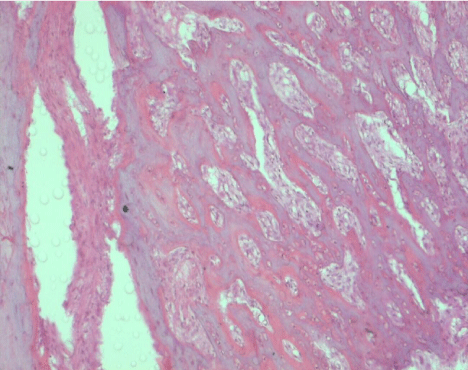
Figure 5. Histologic image of the extraction sockets at day 14 in the LLLT+PRF group (H&E, ×20)
Statistical evaluation of new bone formation
There was a statistically significant difference between the groups in terms of bone amount on the 7th day (p<0.05). The bone amount on the 7th day in the control group was significantly lower than Laser + Platelet-rich fibrin group (Table 1).
Table 1. Results of the Kruskal-Wallis H Test of the Inter-Group Difference in terms of the Bone Amount on Day 7
Since inflammation, granulation tissue formation, angiogenesis, extracellular matrix deposition and maturation stages of the hard tissue healing can be monitored in a coordinated manner during bone healing process after tooth extraction, socket healing is a good experimental model [9]. Therefore, in this study, in order to observe the effect of platelet-rich fibrin and low-level laser therapy, tooth extraction wound was chosen as the experimental model.
Wound healing has been performed experimentally on many different animal models such as guinea pig, dog, rabbit, and rat [10-16]. Hamad et al. (13) has used rabbits in their study where the effects of diode laser on the healing of tooth extraction wound were investigated. Manrique et al. [17], Gau et al. [18], Silva et al. [19] used rats in their studies. In general, rats have been preferred in the majority of the studies on the healing of tooth extraction wound. The studies have shown that socket healing process in rats is similar to that in humans and due to their high metabolic rate, results can be obtained in a short time [20]. In light of the information from the available literature, rat model was chosen in our study.
In the literature, healing of the extraction sockets in rats ranges was monitored between periods of 1-60 days. Healing period after tooth extraction in rats occurs in three phases: early phase (1-5 days) during which the blood clot organization is completed and socket is coated with epithelium, bone formation phase (5-20 days), remodelization phase (20-60 days) during which immature bone matures and alveolar cretin is reshaped [21]. In tooth extraction studies performed in rats, it has been reported that healing of the maxillary incisor socket is completed between 3-6 weeks [22-24]. Considering the information in the literature, sacrifice days in our study were determined as 7th, 10th and 14th days in order to obtain early-stage results of bone healing.
Park et al. [10] has studied the effect of GaAlAs (980 nm) diode laser on wound healing after tooth extractiob in diabetic and healthy rats. They have removed right and left maxillary first molars of all rats and left the extraction sockets on the left side to heal whereas they applied laser to the extraction sockets on the right side at a dose of 13.95 J/cm2 for 60 seconds on the 3rd, 5th, 7th and 14th days. After histological examinations and gene expression analyses, they have reported that the first steps of healing in the extraction sockets exposed to LLLT were observed more rapidly in healthy and diabetic rats, and higher amount of alveolar bone formation was observed compared with the control group. In our study, based on the histological findings obtained on the 7th, 10th and 14th days, higher amounts of bone formation were observed in groups exposed to LLLT than the control groups.
Korany et al. [12] has investigated the effects of LLLT on bone healing in rats that underwent tooth extraction following radiotherapy. They have extracted right and left mandibular first molars on the 3rd day following radiotherapy (6 Gray) and left the extraction sockets on the right side to heal and applied 75mW GaAlAs (830 nm) diode laser to the extraction sockets on the left side. Socket healing was histologically and histomorphometrically analysed on the 3rd, 7th and 10th days. On the 3rd day, they reported the presence of granulation tissue consisting of vascular fibrous tissue at the center of the socket and new woven bone structure at the base of the socket in the laser group, whereas the socket was filled with connective tissue consisting of immature collagen fiber bundles in the group not exposed to laser. On the 7th day, it was stated that a large part of the socket was filled with large bone trabeculae in the laser group, whereas the socket was partially filled with new woven bone tissue in the group not exposed to laser. On the 10th day, it was stated that the woven bone structure transformed into mature bone structure in the laser group. Researchers reported that, on the 7th and 10th days, bone trabeculae percentage increased in the groups exposed to laser compared with the groups not exposed to laser.
Rocha Júnior et al. [25] has applied GaAs (870 nm) diode laser at a daily dose of 3.8 J/cm2 to skin wounds created using punch on the backs of Wistar rats. After applying LLLT 3 sessions per week (on the day of the operation, postoperative day 2 and postoperative day 7), subjects were sacrificed on the postoperative day 10. After histopathological and histomorphometric analysis, more new blood vessel formation, higher fibroblast proliferation and less inflammatory cells were observed in the laser group than the control group on the 10th day. Researchers have concluded that, due to more rapid and more organized healing process, LLLT can be an effective method in tissue repair.
So far, many studies have been conducted to evaluate wound healing in extraction sockets using PRF [26-30]. However, in the literature, there aren’t any studies that evaluate the effects of the combined use of PRF and LLLT on the healing of extraction sockets.
Although centrifugation at 3000 rpm for 10 minutes was recommended to obtain PFR from blood samples collected from rats by many studies in the literature, PRF could not be obtained using these parameters in our preliminary studies. Unlike other studies, Abdullah [31] has obtained PRF by centrifugation at 3000 rpm for 12 minutes. In our study, PRF was obtained by centrifuging 4 ml of blood collected from rats at 3000 rpm for 12 minutes. It was found that this protocol allowed for more PRF to be obtained.
El-Hayes et al. [32] studied the effect of the combined use of LLLT and PRF on bone healing in rabbits. They have created bone cavities in rabbit femurs and divided the subjects into four groups, which were control, LLLT, PRF and LLLT + PRF group. LLLT was applied at a daily dose of 4 J/cm2 using GaAlAs (808 nm) diode laser. Subjects have been sacrificed on postoperative day 30. In their histological analysis, they have found that new bone area was significantly higher in the LLLT + PRF group than the other three groups, and in the LLLT group than the PRF group. Researchers have reported that while LLLT stimulates bone healing, PRF acts much faster. Moreover, they have concluded that when LLLT and PRF are used in combination, they stimulate bone healing more than when they are used alone. In our study where we evaluated bone healing in extraction socket, similarly, we found that the amount of new bone in the group exposed to the combination of LLLT and PRF was significantly higher than the control group on the 7th and 14th days, and than the control and PRF groups on the 10th day.
Nagata et al. [33] have studied the effects of the combined use of PRP and LLLT on the healing of periodontal fenestration defects in rats. Fenestration defects were created in the mandibulae of 80 Wistar rats and the subjects were divided into 4 groups, which were control, PRP, LLLT and PRP + LLLT group. LLLT was performed using InGalP diode laser (660 nm) at a daily dose of 6 J/cm2 (0.035 W, for 5 seconds, to an area of 0.0283 cm2). Subjects were sacrificed on postoperative days 10 and 30. New bone percentage, new bone density, new cement formation and the remaining defect area were analysed histomorphometrically. The remaining defect area on the 10th day in the PRP group was statistically significantly smaller than the control group. New bone percentage and density on the 30th day in the PRP group was statistically significantly higher than the control group. Researchers have concluded that LLLT, PRP, or their combination promoted new cement formation together with functional periodontal ligament, and have reported that the combined use of PRP and LLLT did not have an additional effect compared with their use alone.
Although the application of LLLT alone following tooth extraction accelerates bone healing, the amount of new bone formation reaches to the same level with the control and PRF groups on the 14th day of healing. It was concluded that the combined use of PRF and LLLT stimulates bone healing more than their use alone and increases the volume of the regenerated bone.
- Çirak E, Özyurt A, Peker T, Ömeroglu S, Güngör MN (2018) Comparative evaluation of various low-level laser therapies on bone healing following tooth extraction: An experimental animal study. J Craniomaxillofac Surg. 46: 1147-1152.
- Khadra M (2005) The effect of low level laser irradiation on implant-tissue interaction. In vivo and in vitro studies. Swed Dent J Suppl 172: 1-63.
- Baser Keklikci H, Yagci A, Yay AH, Goktepe O (2020) Effects of 405-, 532-, 650-, and 940-nm wavelengths of low-level laser therapies on orthodontic tooth movement in rats. Prog Orthod 21: 43.
- Dragonas P, Katsaros T, Avila-Ortiz G, Chambrone L, Schiavo JH, Palaiologou A (2019) Effects of leukocyte-platelet-rich fibrin (L-PRF) in different intraoral bone grafting procedures: a systematic review. Int J Oral Maxillofac Surg 48: 250-262.
- Massimo Del Fabbro, Cristina Bucchi, Alessandra Lolato, Stefano Corbella, Tiziano Testori, Silvio Taschieri (2017) Healing of Postextraction Sockets Preserved With Autologous Platelet Concentrates. A Systematic Review and Meta-Analysis. J Oral Maxillofac Surg 75: 1601-1615.
- Thalaimalai DBR, Victor DJ, Prakash PSG, Subramaniam S, Cholan PK (2020) Effect of Low-Level Laser Therapy and Platelet-Rich Fibrin on the Treatment of Intra-bony Defects. J Lasers Med Sci 11: 456-463.
- Vidal B, Pinto A, Galvão MJ, Santos AR, Rodrigues A, Cascão R, Abdulghani S, Caetano- Lopes J, Ferreira A, Fonseca JE, Canhao H (2012) Bone histomorphometry revisited. Acta Reumatol Port 37: 294-300.
- Sargolzaei Aval F, Arab MR, Sargolzaei N, Barfrushan S, Mir M, Sargazi GH, Sargolzaeiaval F, Arab M (2019) Histomorphometric Analysis of Newly-formed Bone Using Octacalcium Phosphate and Bone Matrix Gelatin in Rat Tibial Defects. Arch Bone Jt Surg 7: 182-190.
- Kanyama M, Kuboki T, Akiyama K, Nawachi K, Miyauchi FM, Yatani H, Kubota S, Nakanishi T, Takigawa M (2003) Connective tissue growth factor expressed in rat alveolar bone regeneration sites after tooth extraction. Archives of Oral Biology 48: 723-730.
- Park JJ, Kang KL (2012) Effect of 980-nm GaAlAs diode laser irradiation on healing of extraction sockets in streptozotocin-induced diabetic rats: a pilot study. Lasers Med Sci 27: 223–230.
- Park JB, Ahn SJ, Kang YG, Kim EC, Heo JS, Kang KL (2015) Effects of increased low-level diode laser irradiation time on extraction socket healing in rats. Lasers Med Sci 30: 719–726.
- Korany NS, Mehanni SS, Hakam HM, El-Maghraby EM (2012) Evaluation of socket healing in irradiated rats after diode laser exposure (histological and morphometric studies). Arch Oral Biol 57: 884-891.
- Hamad SA, Naif JS, Abdullah MA (2016) Effect of diode laser on healing of tooth extraction socket: an experimental study in rabbits. J Maxillofac Oral Surg 15: 308-314.
- Asvanund P, Chunhabundit P (2012) Alveolar bone regeneration by implantation of nacre and B-tricalcium phosphate in guinea pig. Implant Dent 21: 248-253.
- Kim KA, Choi EK, Ohe JY, Ahn HW, Kim SJ (2015) Effect of low-level laser therapy on orthodontic tooth movement into bone-grafted alveolar defects. Am J Orthod Dentofacial Orthop 148: 608-617.
- Fronza B, Somacal T, Mayer L, de Moraes JF, de Oliveira MG, Weber JB (2013) Assessment of the systemic effects of low-level laser therapy (LLLT) on thyroid hormone function in a rabbit model. Int J Oral Maxillofac Surg 42: 26-30.
- Manrique N, Pereira CC, Garcia LM, Micaroni S, Carvalho AA, Perri SH, Okamoto R, Sumida DH, Antoniali C (2012) Alveolar bone healing process in spontaneously hypertensive rats (SHR). A radiographic densitometry study. J Appl Oral Sci 20:222-227.
- Gau CH, Hsieh YD, Shen EC, Lee S, Chiang CY, Fu E (2005) Healing following tooth extraction in cyclosporine-fed rats. Int J Oral Maxillofac Surg 34: 782-788.
- Silva HC, Coletta RD, Jorge J, Bolzani G, De Almeida OP, Graner E (2001) The effect of cyclosporin A on the activity of matrix metalloproteinases during the healing of rat molar extraction wounds. Archives of oral biology 46: 875-879.
- Elsubeihi ES, Heersche JNM (2004) Quantitative assessment of postextraction healing and alveolar ridge remodelling of the mandible in female rats. Archives of Oral Biology 49: 401-412.
- Pietrokovski J, Massler M (1967) Ridge remodelling after tooth extraction in rats. J Dent Res 46: 222-231.
- Okamoto T, Russo MC: Wound healing following tooth extraction (1973) Histochemical study in rats. Rev Fac Odont Araçatuba 2: 153-164.
- Santos Jr PV, Melhado RM (1990) Efeitos da estimulação ultra-sônica sobre o processo de reparo em ferida de extração dental: estudo histológico em ratos. Rev Odont Unesp 19: 291-299.
- Carvalho TLL, Bombonato KF, Brentegani LG (1997) Histometric analysis of rat alveolar wound healing. Braz Dent J 8: 9-12.
- Rocha Junior AM, Oliveira RG, Farias RE, Andrade LCF, Aarestrup FM (2006) Modulation of fibroblast proliferation and inflammatory response by low-intensity laser therapy in tissue repair process. An Bras Dermatol 81: 150-156.
- Simon B, Zatcoff A, Kong JJ, O’Connell S (2009) Clinical and histological comparison of extraction socket healing following the use of autologous platelet-rich fibrin matrix (PRFM) to ridge preservation procedures employing demineralized freeze dried bone allograft material and membrane. The Open Dentistry Journal 3: 92-99.
- Marenzi G, Riccitiello F, Tia M, di Lauro A, Sammartino G. Influence of leukocyte- and platelet-rich fibrin (L-PRF) in the healing of simple postextraction sockets: a split-mouth study (2015) BioMed Research International 369273: 6.
- Anwandter A, Bohmann S, Nally M, Castro AB, Quirynen M, Pinto N (2016) Dimensional changes of the post extraction alveolar ridge, preserved with leukocyte- and platelet rich fibrin: a clinical pilot study. J Dent 52: 23-29.
- Hatakeyama I, Marukawa E, Takahashi Y, Omura K (2014) Effects of platelet-poor plasma, platelet-rich plasma, and platelet-rich fibrin on healing of extraction sockets with buccal dehiscence in dogs. Tissue Eng Part A 20: 874-882.
- Girish Rao S, Bhat P, Nagesh KS, Rao GH, Mirle B, Kharbhari L, Gangaprasad B (2013) Bone regeneration in extraction sockets with autologous platelet rich fibrin gel. J Maxillofac Oral Surg 12: 11-16.
- Abdullah WA (2016) Evaluation of bone regenerative capacity in rats claverial bone defect using platelet rich fibrin with and without beta tri calcium phosphate bone graft material. Saudi Dent J 28: 109-117.
- El-Hayes KA, Zaky AA, Ibrahim ZA, Allam GFA, Allam MF (2016) Usage of low level laser biostimulation and platelet rich fibrin in bone healing: experimental study. Dent Med Probl 53: 338–344.
- Nagata MJH, Campos N, Messora MR, Pola NM, Santinoni CS, Bomfim SRM, Fucini SE, Ervolino E, Almeida JM, Theodoro LH, Garcia VG (2014) Platelet-rich plasma, low-level laser therapy or their combination promote periodontal regeneration in fenestration defects: a preliminary in vivo study. J Periodontol 85: 770-78.





