Diabetes is a complex disease that principally arises from a failure of function in the pancreatic islet beta cell. Intensifying studies in human islets will accelerate development of clinically relevant therapeutics. Access to human islets is limited and compounded because human islets loose function rapidly and do not survive beyond a few days in current culture conditions. During islet isolation disruption of the islet microenvironment and loss of trophic support subject islets to cellular stress that impairs islet function and survival. A human placenta-derived hydrogel has been evaluated for support of human islet function in culture. Human islets from non-diabetic and type 2 diabetic donors were studied and beta cell function assessed by stimulated insulin secretion. Human islets from non-diabetic donors showed enhanced insulin secretion after exposure to the hydrogel with 291% increase in insulin secretion. Human islets from type 2 diabetic donors remained mechanistically responsive to the hydrogel having enhanced insulin secretion. Exposure to a human placenta-derived hydrogel offers a new paradigm to enhance human islet function in ex vivo culture.
pancreatic islet, hydrogel
Diabetes is an evolving worldwide health concern. While it is a complex disease, diabetes principally arises from a failure of function in the pancreatic islet beta cell. The biology of human islets is not fully recapitulated in animal studies, identifying a need for direct study of human islets. Access to human islets is limited and compounded since human islets loose function rapidly and do not survive beyond a few days when cultured ex vivo [1,2].
Development of new modalities to culture human islets to promote longer term survival and function is required.
Extracellular matrix (ECM) is an important component of the islet microenvironment that is lost during islet isolation. ECM is a complex of different molecules that serve as cellular scaffold, and factors that signal to regulate survival, function and differentiation. ECM is an essential component for the maintenance of cell homeostasis [3-5]. Better understanding of the interaction between pancreatic islets and their local microenvironment could contribute to advances in diabetic therapies [6,7]. The ability to preserve ECM-human islet interaction may enhance islet culture techniques to promote survival and maintenance of functional islets.
Improved islet and beta cell survival and function is seen when cultured on ECM-derived substrates, including cell-secreted matrices and individual purified ECM proteins [4,5,8]. Promoting cell expansion of adult islets cells in vitro has proven a challenge in all species [9,10]. Plasticity amongst pancreatic cells has been reported resulting in islet/beta-like cells (11). Adult pancreatic duct cells are a potential source of islet cells when provided with the correct differentiation cues, including ECM (10,12,13,14). A limitation of most ECM substrates is they originate from non-human sources, which restricts their clinical application.
Pancreatic islet transplantation has reversed type 1 diabetes in a limited number of patients [15,16]. During islet isolation and pre-transplantation, the destruction of the islet microenvironment and the loss of trophic support subject islets to cellular stress that impairs islet function and survival [1,2,17]. Culture parameters that enhance the health and survival of isolated human islets would have great benefit for clinical islet transplantations. In the current study, a novel human hydrogel is evaluated. HuECM is a complex ECM derived from human placenta a highly enriched and readily available tissue source. Its potential to provide support to human islet function has been evaluated by glucose induced insulin secretion in human islets from diabetic and non-diabetic donors.
Ethics
Protocols and procedures were reviewed and approved by relevant institutional regulatory committees.
Human extracellular matrix (HuECM)
Human Extracellular Matrix (HuECM) was provided by Life Net Health, Inc., Virginia Beach, VA. This product, derived from human placenta, provides a natural structural support for cells attachment and growth as well as an enhanced growth factor-rich environment to support cell activity in vitro. HuECM is a unique complex collagen- based matrix material for in vitro applications. The protein profile is tissue–specific and includes collagens (I, IV, VI, XII), elastin, chondroitin and heparin sulfate proteoglycan, laminin and entactin, long with vascular endothelial growth factor (VEGF A & B), platelet-derived growth factors (PDGFs), insulin-like growth factor (IGF-2), hepatocyte growth factor/scatter factor (HGF/SF), epidermal growth factor (EGF), transforming growth factors (TGF), and basic fibroblast growth factor (bFGF). The appropriate hydrogel conditions (i.e. gel concentration, stiffness and matrix volume) and hydrogel formats (i.e. thin coating, thin gel, thick gel) are application dependent. Lower concentrations of HuECM will form a delicate gel that creates a sheath around each islet.
Culture of human islets
Human islets from non-diabetic and type 2 diabetic donors were obtained from the Integrated Islet Distribution Program or Prodo Laboratories. The islets were incubated overnight in CMRL-1066 (Corning, Cellgro Manassas, VA), medium supplemented with 10% FBS (Gibco, Grand Islands NY)) and 1% penicillin-streptomycin (Gibco, Carlsbad, CA) at 37˚C in 5% CO2 to recover from shipment. After overnight culture, they were placed into a 12 well suspension culture plate (CellStar, Fisher Scientific, Nazareth, PA) with CMRL-1066 + 1% FBS+ 1% penicillin-streptomycin and then cultured either as a suspension (media only) or cultured with HuECM at the stated concentration. The culture time varied from 24 to 48 hour depending on the experiment design. The HuECM in culture at lower concentration creates a delicate spider-like sphere around the islets and they are naturally suspended in medium. The in vitro 3D culture aims to mimic the in vivo microenvironment and creates a relatively solid structure in which the islets remain without the ability to move [18].
Glucose stimulated insulin secretion (GSIS)
Perifusion: The perifusion experiments were performed using a BioRad Econo Gradient Pump connected to Biorad Biologic BioFrac Fraction Collector [19]. Measured was the insulin secretion rate of 200 human non-diabetic islets cultured in suspension or with 0.5 mg/ml matrix for 24 hr. After 24 hr culture, islets were handpicked and applied to a column. Islets cultured with matrix where separated from matrix by gentle spinning before applying to column. Two different perifusion buffers were used; perifusion buffer A (114 mM NaCl, 5 mM KCL, 24 mM NaHCO3,1 mM MgCl2.6H2O and 2.2 mM CaCl22H2O, 10 mM HEPES, 0.83 mM Na2HPO4, 0.25% BSA and glucose 3 mM glucose, pH 7.4) and perifusion buffer B (150 ml of buffer A with 23 mM glucose). After a 45 min washing step with low glucose (3 mM) for stabilization, islets were stimulated with the following sequence: 5 min of low glucose, 30 min of high glucose (23 mM) and 15 min low glucose. Samples were collected every minute: 1 ml/min flow rate, 200 IEQ per column. Insulin secretion was determined by insulin ELISA following manufacturer’s directions (Mercodia, Winston-Salem, NC). Total islet insulin content was measured by ELISA after extraction from acidified ethanol. The results were also normalized to the total insulin content, this did not change interpretation of the data (not shown).
Static: GSIS was performed using the Krebs-Ringer buffer (KRB) [20]. After 24 hr incubation in CMRL-1066 + 1%FBS + 1% penicillin-streptomycin, islets were transferred to a 12 well suspension culture plate containing 1 ml KRB without glucose and were incubated for 1 hr at 37˚C in 5% CO2. Then glucose was added to designated samples to reach the concentration of 1 mM and 18 mM and incubated for 1 hour at 37˚C in 5% CO2. Each condition was performed in triplicate. After incubation, supernatant was collected for determination of insulin secretion by human insulin-linked immunosorbent assay ELISA (Mercodia, Winston- Salem, NC) following manufacturers directions.
Dithizone (DTZ) staining
Dithizone (DTZ) (Sigma-Aldrich, St. Louis, MO) stock solution was prepared by dissolving 50 mg of DTZ in 5 ml of dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO) and incubated briefly at 20˚C. Islets were cultured 24 hours in 12 well suspension plates in CMRL-1066 + 1% penicillin-streptomycin + 1% FBS only (control) or in the same medium with stated concentrations of HuECM. Staining with DTZ was performed by adding 10 µl of filtered stock solution to 1 ml of islets culture medium and incubating at 37℃ for 15 min.
Following three washes with Hank’s Balanced Salt Solution (HBSS, Gibco, Life technologies, Grand Islands, NY) the crimson red-stained cell clusters were examined under an inverted microscope (Olympus IX73 and CS Cell SensSoftware, Japan).
Statistics: The data are presented as means ± SEM and were analyzed using either Two Way ANOVA or the t-Test (Prism 5.0; Graph-Pad Software, La Jolla, CA). Values of p<0.05 are considered significant.
Incubation models to evaluate HuECM on human islet function
Human extracellular matrix (HuECM) is a protein-rich matrix derived from human placenta. The ability of HuECM to form a hydrogel allows exploration of protein factors in a defined physical support structure. Islets, in vivo, are embedded within the pancreas and derive signals from protein factors and extracellular dimensional organization. Upon isolation, islets loose these molecular cues that may contribute to their decline in function and poor survival in vitro. The HuECM provided at least three paradigms for investigation. Depending on HuECM concentration within culture, the hydrogel formation and density can be adjusted. The cartoon (Figure 1) shows three potential paradigms to evaluate the utility of HuECM for preservation of islet function in vitro. Studies with hydrogel in paradigms illustrated in Figures 1C and 1D are under ongoing investigation. In this brief report, the effects of HuECM on human islet function, when added as a supplement is explored (Figure 1).
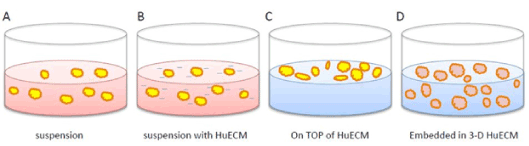
Figure 1. Schematic diagram of islet culture paradigms using human placenta-derived hydrogel (HuECM)
Depicted are control conditions for suspension culture (A), low supplemental concentrations (0.0625 – 0.5 mg/ml) of HuECM (B), higher concentration of huECM (>0.5 mg/ml) with islets incubated on top of gel (C) or embedded within the gel (D).
Effect of HuECM exposure on human islet function
Function of human donor islets was assessed by measuring glucose stimulated insulin secretion (GSIS). Upon a change in glucose tone, healthy islet beta cells respond by releasing stored insulin into the surrounding environment. Four human donor islet preparations were assessed. Human islets were hand picked and cultured for 24 hrs in the absence or presence of 0.5 mg/ml HuECM before being assessed by perfusion GSIS (Figure 2).
Following a period of equilibration, islets were pulsed with elevated glucose (23 mM). Fractions were collected at one-minute intervals and measured for insulin by ELISA. Islets treated with and without matrix achieved stable baselines that were not significantly different when equilibrated with a basal glucose concentration (3 mM). Insulin was increased upon transition to 23 mM glucose the concentration of which continued to rise before reaching a peak at four minutes. Relative to control, HuECM exposed islets showed a significant elevation in insulin peak (p<0.05) and an overall significantly elevated insulin release measured by AUC; 1426 ± 423 vs 4159 ± 720 for control vs HuECM respectively (p<0.01, Figure 2).
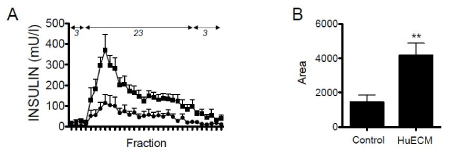
Figure 2. Functional assessment of islets from non-diabetic donors
In A, islets were incubated with 0.5 mg/ml HuECM (squares) or without (circles) for 24-hour culture and function assessed by perifusion. Islets were perfused with low (3 mmol/l) and high (23 mmol/l) glucose. The AUC between the two treatment conditions are shown (B). Data shown is from four donor preparations, **p<0.01
Human donor islet responses were assessed after 48-hour exposure to HuECM relative to islets from the same donors that had been cultured for the same time period but had not been exposed to 0.0625 mg/ml HuECM. Three donors were studied in a static GSIS model (Figure 3). Transfer from basal 1 mM glucose to 18 mM glucose induced a significant increase in insulin secretion for both groups. Human islets that had been exposed to HuECM had significantly elevated insulin secretion at both basal and stimulated glucose concentrations (p<0.05).
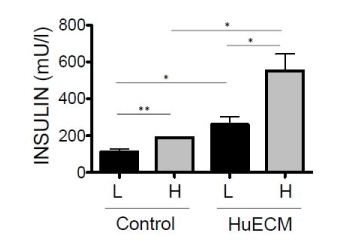
Figure 3. Glucose stimulated insulin secretion of human islets from non-diabetic donors
Insulin secretion was determined in islets cultured in the absence (control) or presence of 0.0625mg/ml HuECM for 48 hours. Islets were stimulated with low 1mM glucose (L, black bars) or high 18mM glucose (H, grey bars) and insulin secretion measured by ELISA. Data shown is from three donor islet preparations, *p<0.05 and **p<0.01.
Human islets from type 2 donors have enhanced function upon exposure to HuECM
Islets from type 2 diabetic donors characteristically have a diminished insulin secretion response to a pulse of elevated glucose. A limited study has been performed in islets obtained from type 2 diabetic donors to assess if HuECM exposure also boosts the glucose- stimulated insulin response. In the static GSIS paradigm, five preparations of islets obtained from human donors with type 2 diabetes was assessed. The GSIS in human islets from type two diabetic donors that had been exposed to 0.0625 mg/ml HuECM showed a significantly higher basal and stimulated release of insulin (Figure 4). Human islets were also assessed for GSIS response following incubation conditions that embedded the islets in 1 mg/ml HuECM to provide a 3-dimensional (3-D) interaction (Figure 1). While some variation in the magnitude of donor response was observed a clear trend for elevated insulin secretion resulted (Figure 4). Penetration of nutrients into the 3-D matrix was confirmed by dithizone uptake (data not shown). Accumulation of dithizone was equivalent to control islets.
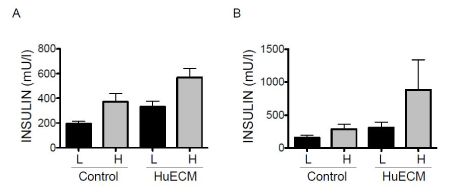
Figure 4. Glucose stimulated insulin secretion of human islets from type 2 diabetic donors
In A, insulin secretion in islets cultured in the absence (control) or presence of 0.0625mg/ml HuECM for 24 hours was measured with low glucose (L, black bars) or high glucose (H, grey bars). Insulin secretion in response to glucose was assessed in islets grown embedded three dimensionally in 1mg/ml HuECM for 24 hours (B), *p<0.05.
A rapid decline of islet viability and loss of stimulus-induced insulin secretion remains a major challenge for investigations involving primary human pancreatic islets in culture [1,2,16]. In the past several years, recapitulation of embryonic cues has stimulated investigation of a variety of 3-D cell culture systems. It is clear that 3-D culture systems hold great promise for applications in drug discovery, cancer biology, stem cell research and many other cell-based analyses and devices. Evolving evidence demonstrate that 3-D cell culture systems provide more holistic models than the traditional 2D monolayer culture [3,4,18,21]. Recent studies in stem cell research indicate extracellular matrix (ECM) as a key component for the maintenance of a cell’s state of differentiation [22-24].
This study has investigated a human placenta-derived extracellular matrix (HuECM) to evaluate if human islet interaction with HuECM preserves beta cell function. The results show that HuECM has a positive protective effect on islet function. Stimulated insulin secretion in islets from non-diabetic donors exposed to HuECM had a significantly elevated insulin release following glucose challenge. Importantly, islets from type 2 diabetic donors also showed a significant positive increase in stimulated insulin after exposure to HuECM. Dysfunctional glucose simulated insulin secretion is a characteristic feature of type 2 diabetes. It is highly encouraging, for future anti-diabetic strategies, that islets from type 2 diabetic donors have the molecular machinery preserved to be responsive to stimuli provided by HuECM. While additional investigation is required, the observation that HuECM in a 3-D paradigm further enhanced insulin secretion in type 2 donor islets is noteworthy. The higher concentration of HuECM to enable a 3-D culture also increases the relative concentration of HuECM soluble factors. Dissection is required of the relative contribution between HuECM soluble factors and HuECM structural support or induced synergy between both.
HuECM is a human placenta derived complex collagen-based material containing connective tissue proteins and growth factors. In addition to the advantage of species compatibility, the placental growth factors are candidate elements to support long-term survival and/or differentiation of human islets ex vivo, especially in the context of three dimensional extracellular support. Studies are ongoing and supported by long-term survival of islets, retention of insulin staining and, neo-expression of the differentiation marker CK19 in islets exposed to HuECM relative to control (DS unpublished results) [25].
Methodologies to retain human islet function and survival ex vivo would significantly enhance research focused on diabetes and facilitate entry of new disciplines by removing barriers to tissue access. Sustained access to high functioning human islets will, at minimum, progress knowledge on islet pathophysiology, accelerate new drug discovery and advance use of islets in human transplantation. To date, human islet transplantation is the only recognized strategy that has reversed type 1 diabetes. The success of human islet transplantation is critically limited by islet access and maintenance of islet function [15]. Novel human ECMs will likely be key additions to new methodologies for islet survival in culture. This report on enhanced human islet function in culture following exposure to human placenta derived extracellular matrix (HuECM) is an exciting new lead into this arena.
Grateful to LifeNet Health, Inc., of Virginia Beach, VA for providing HuECM.
Grant support was provided by Commonwealth Research Commercialization Fund.
- Paraskevas S, Maysinger D, Wang R, Duguid TP, Rosenberg L (2000) Cell loss in isolated human islets occurs by apoptosis. Pancreas 20: 270-276. [Crossref]
- Paraskevas S, Duguid WP, Maysinger D, Feldman L, Agapitos D, et al. (1997) Apoptosis occurs in freshly isolated human islets under standard culture conditions. Transplant Proc 29: 750-752. [Crossref]
- Wang RN, Rosenberg L (1999) Maintenance of beta-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship. J Endocrinol 163: 181-190. [Crossref]
- Weber LM, Hayda KN, Anseth KS (2008) Cell-Matrix Interactions improve b-cell survival and insulin secretion in three-dimensional culture. Tissue Eng Part A 14: 1959-1968.
- Riopel M, Wang R (2014) Collagen matrix support of pancreatic islet survival and function. Front Biosci (Landmark Ed) 19: 77-90. [Crossref]
- Lysy PA, Weir GC, Bonner-Weir S (2012) Concise review: pancreas regeneration: recent advances and perspectives. Stem Cells Transl Med 1: 150-159.
- Puddu A, Sanguineti R, Mach F, Dallergi F, Viviani GL, et al. (2013) Update on the protective molecular pathways improving pancreatic beta-cell dysfunction. Mediators Inflamm 5: 1-10.
- Lucas Clerc C, Massart C, Campion JP, Launois B, Nicol M (1993) Long-term culture of human pancreatic islets in an extracellular matrix: morphological and metabolic effects. Mol and Cell Endocrinol 94: 9-20
- Bonner Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, et al. (2000) In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci USA 97: 7999-8004. [Crossref]
- Brelje TC, Scharp DW, Lacy PE, Ogren L, Talamantes F, et al. (1993) Effect of homologous placental lactogens, prolactins and growth hormones on islets b-cell division and insulin secretion in rat. Mouse and human islets: implication for placental lactogen regulation of islets functioning during pregnancy. Endocrinology 132: 879-887.
- Taylor-Fishwick DA, Pittenger GL (2010) Harnessing the pancreatic stem cell. Endocrinol Metab Clin North Am 39: 763-776. [Crossref]
- Bonner Weir, Smith FE. (1997) Pancreatic growth and regeneration ed. Sarvetnick N (Karger, New York) pp: 138-153.
- Streuli C (1999) Extracellular matrix remodelling and cellular differentiation. Curr Opin Cell Biol 11: 634-640. [Crossref]
- Hulinsky I, Harrington J, Cooney S, Silink M (1995) Insulin secretion and DNA synthesis of cultured islets of Langerhans are influenced by the matrix. Pancreas 11: 309-314. [Crossref]
- Taylor-Fishwick DA, Pittenger GL, Vinik AI (2008) Transplantation and Beyond. Drug Devel Res 69: 165-176. [Crossref]
- Noguchi H, Miyagi-Shiohira C, Kurima K, Kobayashi N, Saitoh I, et al. (2015) Islet Culture/Preservation Before Islet Transplantation. Cell Med 8: 25-29. [Crossref]
- Bissell MJ (2016) Thinking in three dimensions: discovering reciprocal signaling between the extracellular matrix and nucleus and the wisdom of microenvironment and tissue architecture. Mol Biol Cell 27: 3205-3209. [Crossref]
- Edmondson R, Broglie JJ, Adcock AF, Yang L (2014) Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol 12: 207-218. [Crossref]
- Ma K, Xiao A, Park SH, Glenn L, Jackson L, et al. (2017) 12-Lipoxygenase Inhibitor Improves Functions of Cytokine-Treated Human Islets and Type 2 Diabetic Islets. J Clin Endocrinol Metab 102: 2789-2797. [Crossref]
- Taylor-Fishwick DA, Weaver JR, Grzesik W, Chakrabarti S, Green-Mitchell S, et al. (2013) Production and function of IL-12 in islets and beta cells. Diabetologia 56: 126-135. [Crossref]
- Chen C, Cohrs CM, Stertmann J (2017) Human beta cell mass and function in diabetes: Recent advances in knowledge and technologies to understand disease pathogenesis. Mol Metab 6: 943-957. [Crossref]
- Gattazzo F, Urciuolo A, Bonaldo P (2014) Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta 1840: 2506-2519. [Crossref]
- Bonnans C, Chou J, Werb Z (2014) Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15: 786-801. [Crossref]
- Jacobson EF, Tzanakakis E. (2017) Human pluriopotent stem cell differentiation to functional pancreatic cells for diabetes therapies: Innovation, challenges and future directions. J Biol Eng 11: 21-29.
- Corritore E, Dugnani E, Pasquale V, Misawa R, Witkowski P, et al. (2014) β-Cell differentiation of human pancreatic duct-derived cells after in vitro expansion. Cell Reprogram 16: 456-466. [Crossref]




