Abstract
Melanin is the most recognised pigment involved in the pigmentation of human skin. It serves as a natural UV protectant, preventing DNA damage. Melanin is produced in the melanosomes (following stimulation by various causes, such as UV rays and free radicals), by molecular regulatory means involving, among others, the enzyme tyrosinase. Tyrosinase inhibitors are present in many plants, offering a natural solution for hyperpigmentation disorders. OceanDerMX® Balance & Brighten contains four ingredients, two of them, Centella asiatica (Gotu Kola) and Glycyrrhiza glabra (Liquorice root), are known to reduce the melanin formation through different modes of action; anti-inflammatory for Gotu Kola [1-4], and tyrosinase inhibition for Liquorice [3,5,6]. The present study intends to determine the clinical tolerance and efficacy of a cosmetic formula containing 5% of OceanDerMX® Balance & Brighten in lightening the skin and improving brightness in 27 healthy Asian women over 56 days of use twice a day. The results show that OceanDerMX® Balance & Brighten at 5% is effective in improving skin brightness and reducing pigmentation in as little as 14 days. Self-evaluation of the subjects also revealed a perceived efficacy. No indication of serious adverse effects following 56 days of use was reported.
Key words
pigmentation, brightening, tyrosinase, inhibitor, clinical efficacy, bio-active, botanicals, skincare, cosmetics, plant extracts, in vitro, in vivo
Introduction
No two people have the same distribution of pigment throughout their body, leading to unique placements of sunspots and pigmentation [7]. Perhaps, the most recognised primary pigment involved in skin pigmentation is melanin which is produced by melanocytes during melanogenesis [8].
Although pigmentation is inheritable through its genetic and endocrinal properties, environmental factors also play a role in melanin’s regulation [7]. Among heat regulation and camouflage, melanin also serves to protect the skin from UV radiation using a defence system [7]. The skin reacts to internal and external factors by modifying the constituent pattern of pigmentation [7]. Melanin is said to act as a natural sunscreen by neutralising the reactive oxygen species (ROS) that can damage cells [7]. Melanocytes transfer melanosomes to keratinocytes through their dendrites to form melanin caps that reduce DNA damage caused by UV rays in the epidermis [7]. UV rays contribute to hyperpigmentation by increasing the rate of transcription for the TYR gene, responsible for the synthesis of tyrosinase. Tyrosinase is a rate-limiting enzyme involved in the synthesis of melanin [8]. The tyrosinase enzyme in melanocytes converts the amino-acid tyrosine into dopaquinone which enters further chemical reactions to become melanin [8]. Solar lentigines, otherwise known as age spots, are typically found on UV-exposed areas of the body [7].
ITAo |
Skin color classification |
ITAo > 55o |
Very light skin |
55o > ITAo > 41o |
Light skin |
41o > ITAo > 28o |
Intermediate skin |
28o > ITAo > 10o |
Tan skin |
10o > ITAo > -30o |
Brown skin |
-30o > ITAo |
Dark skin |
Table 1. ITAo skin colour classification
A study on hyperpigmentation in cases of solar lentigines uncovered the molecular mechanics, stating there is a 2-fold increase in TYR-positive cells per length of the dermal-epidermal interface of these lesions compared with unaffected skin [7]. As such, it is believed that there is a molecular regulatory network between melanocytes and keratinocytes as well as melanocytes and dermal fibroblasts [7]. This network is responsible for UV rays’ stimulation of melanocytes to produce melanin [7]. Older melanocytes are believed to hold greater functional activity due to the cumulation of sun exposure, explaining why pigmentation tends to increase with age [7].
Thereby, one of the ways to prevent hyperpigmentation is by controlling tyrosinase’s activity through tyrosinase inhibition [8].
The use of botanical ingredients in skincare for their anti-inflammatory and antioxidant properties has occurred for thousands of years [9]. Diverse in polyphenols and flavonoids, many plants offer promising means as a topical treatment for a variety of skin conditions, such as hyperpigmentation. Botanicals are increasingly sought after for their abundance of biological activity, being a natural alternative to synthetic and chemical substances, providing better bioavailability [4,8,10]. Plant extracts entail a plethora of benefits, primarily linked to their antimicrobial activities, such as tyrosinase inhibition [11]. Phenolic compounds comprise the biggest group of phytochemicals in plants and are responsible for the biological activity in plant extracts, being classified as potent tyrosinase inhibitors [8]. Despite the vast range of tyrosinase inhibitors from natural sources, only a few are known to be safe as well as effective [8]. Zolghadri and colleagues (2019) recommend the examination of the safety and efficacy of such inhibitors through in vitro as well as in vivo experiments to better understand their functional mechanisms [8].
Plant formulations begin with extraction. There has been extensive research to find methods that are both efficient and efficacious [12]. Efficiency corresponds to the yield of extraction whereas efficacy is the potency (magnitude of bioactivity) of the extract and its ability to produce an effect [12]. A synergistic strategy for tyrosinase inhibitors may be useful in improving their inhibitory activities, with mixtures of bioactive compounds showing to have an increase in the synergistic effect of tyrosinase [8]. The proprietary method of extraction, for OceanDerMX® products, is a result of eight years of comprehensive research and development and employs an innovative, three-layered, cold-processed and chemical-free bio-liquefaction approach, using ultra-distilled water or organic carrier oil as a single solvent. The focus is to obtain the best possible quality and quantity of cosmeceutical bio-actives derived from fresh New Zealand land and marine botanicals. This unique synchronized alliance through the bio-liquefaction of raw-processed ingredients emphasizes the polysaccharide network between bio-actives, especially in combination with each other. As such, this technology provides a single multitasking input that replaces multiple ingredients, significantly reducing formulating and manufacturing time and cost.
Centella asiatica (Gotu Kola) is a potent natural ingredient used to brighten the skin. Gotu Kola is abundant in asiaticoside, which is believed to inhibit the expression of tyrosinase, reducing pigmentation in the long run [1] [2,5,13]. Since free radicals are also involved in the pigmentation process, the anti-inflammatory and antioxidant activity of Centella asiatica is also important. Glycyrrhiza glabra (Liquorice root) is a highly sought-after skin brightening and depigmentation cosmetic ingredient said to inhibit tyrosinase through mechanisms other than oxidant reducing activity. Instead, the compounds within Liquorice root block the active site of tyrosinase, so it temporarily cannot perform its catalytic activity to produce melanin [3,5,6].
Some New Zealand native plants have developed marvellous biomimetic biopolymers as defensive and adaptive mechanisms to survive and thrive in their unique environment where the thin ozone layer offers little protection against aggressive UV rays. Centella asiatica (Gotu Kola) and Glycyrrhiza glabra (Liquorice root), form a synchronised alliance with New Zealand native red seaweed (Sarcothalia circumcincta) and Cyathea medullaris (Mamaku/Black fern) in OceanDerMX® Balance & Brighten. New Zealand native red seaweed (Sarcothalia circumcincta) is known to have a hydrating and plumping effect on the skin which is attributed to the unique mix of versatile biomimetic sulphated glycosaminoglycans (sGAGs). These natural biopolymers form a fishnet-like matrix that helps hold water molecules, while also acting as a structural scaffold to tighten the skin [10,14,15]. Cyathea medullaris (Mamaku/Black fern) produce mucilage containing Glucuronomannans, which also play a major role in forming the 3D-viscoelastic matrix that can rapidly hydrate. Consequently, this synergistic activity provides an immediate lifting and tightening of the skin [14,16].
Previous research has shown asiaticoside, in Centella asiatica (Gotu Kola), to reduce melanin content in melanocytes as well as suppress tyrosinase mRNA and protein expression in the B16F10 melanoma cells in mice, indicating that asiaticoside may be effective in the treatment of hyperpigmentation or be used for skin brightening [13]. Similarly, bioactive phenols from within Glycyrrhiza glabra (Liquorice root) have also been shown to downregulate tyrosinase, inhibiting melanogenesis in B1610 mouse melanoma cells [17]. The objective of this study is to test the tolerance and the clinical efficacy of OceanDerMX® Balance & Brighten at 5% concentration, for improving the appearance of pigmentation on the facial skin of 27 healthy female Asian volunteers aged 18 and above through standardised photography, instrumental evaluations, and a self-assessed questionnaire. The product was also assessed in vitro for its sulphated glycosaminoglycans (sGAGs) concentration and relative total antioxidant capacity.
Materials and methods
In vitro
sGAGs concentration of OceanDerMX® products: Sulphated glycosaminoglycans (sGAGs) contents of the OceanDerMX® samples were measured by a spectrophotometric method using Alcian Blue dye. sGAGs contents were stained and precipitated with Alcian Blue, and the absorbance of the supernatant was measured at 610 nm in a microplate reader [SPECTROstar nano (BMG Tech)]. The colour of the supernatant is indirectly proportional to the quantity of sGAGs in the sample. This linear relationship was calibrated using Fucoidan with a different range of concentrations as standards.
Relative total antioxidant capacity of OceanDerMX® balance & brighten: Antioxidant activity of different product range samples was determined by CUPRAC (CUPric Reducing Antioxidant Capacity) Method. The reducing activity of potential hydrophilic and lipophilic antioxidants within the product samples was tested. The absorbance of Cu(I)-neocuproine (Nc) chelate in the samples was measured at 450 nm in a plate reader [SPECTROstar nano (BMG Tech)]. Trolox with a different range of concentration was used as a standard.
In vivo
Study design and ethical aspects: This clinical study used an open intra-individual test with each volunteer acting as their own control. A cosmetic cream containing 5% OceanDerMX® Balance & Brighten and no other brightening ingredient was used. As such, the before- and after-use are referred to for comparison. Written informed consent was obtained from all volunteers.
Study participants: A total of 27 healthy Asian females aged 18 and above were recruited for this study. The main exclusion criteria included those that were pregnant or lactating, had a major systemic disease such as cancer or skin disease such as dermatitis and were allergic to cosmetic or preservative products. Volunteers were instructed not to take any oral supplements or use systemic or topical steroids but could continue their regular cosmetic routine as well as use their cleanser of choice. Their moisturiser was swapped for OceanDerMX® Balance & Brighten at 5% and they were instructed to use it twice daily. No serums or other topical treatments, including laser treatment were permitted for the duration of the study.
Study schedule: The study took place in Guangzhou, China, from February 10 to April 7, 2022. The duration of the study was 56 days where volunteers attended visits on D0 (before application), D14 (after 14 days of use) D28 (after 28 days of use), and D56 (after 56 days of use). Volunteers were instructed to apply the product to their full face, twice daily. At each visit, data was collected by the evaluation of pigmentation using the Spectrophotometer® (Minolta CM-700-d) probe and photos were taken using the Visia®-CR, manufactured by Canfield Scientific, Inc in Parsippany, New Jersey, USA. Volunteers were clinically examined by a dermatologist at each visit. The effects of the product were evaluated through a self-assessment questionnaire. Each volunteer was also given a daily log to track the use of the product as well as adverse events.
Test product: The test product is classified as a moisturiser. The face lotion formulation contains 5% of OceanDerMX® Balance & Brighten a special blend of extracts from New Zealand native red seaweed (Sarcothalia circumcincta), Mamaku/Black fern (Cyathea medullaris), Gotu Kola (Centella asiatica) and Liquorice root (Glycyrrhiza glabra).
Evaluation of skin lightening and brightening effects: The pigmentation on each volunteer’s facial skin was evaluated on D0, D14, D28, and D56 by use of a skin profiling instrument, Spectrophotometer® on the forehead and both cheeks. Measurements involved taking three repetitions in a row. Photos were taken of the full face using the Visia®-CR on D0, D14, D28, and D56. All measurements were conducted under rested conditions, involving 15 minutes of acclimatising to the stable physical environmental conditions with a room temperature of 20 degrees Celsius and humidity between 40%-60%.
Subject self-evaluation questionnaire: Volunteers were supplied with a self-evaluation questionnaire to answer on D14, D28, and D56, assessing their view toward a clearer complexion, moisture, smoothness, and radiance of their skin.
Illustration of clinical efficacy: Photos of the volunteers’ faces, including front, right and left profiles, were taken on D0, D14, D28, and D56 using the Visia®-CR skin imaging system, on S2 (standard 2: flat lighting) mode. The Spectrophotometer® was used to evaluate each volunteer’s facial skin on D0, D14, D28, and D56. Images and results were labelled with the subject number, study number, the probe the image/result was taken with, and the filter used.
Statistical analysis: For each quantitative parameter (L*, a*, b* and ITAo), the evolution across time was measured by the percent increase or decrease over the duration of the study (D14, D28 and D56) using the Student’s Paired t-test. Statistical analyses were performed at 5% significance using 2-sided tests.
CIELAB colour space (L*a*b*): The CIELAB colour space (also known as L*a*b*) is a colour space defined by the International Commission of Illumination (CIE). Each letter represents an axis [18]. There is a correlation between the colour space and the human skin:
L* gives information about the black-white axis; the brightness/lightness. The higher the L*, the brighter the skin.
The a* value located on the green-red axis is proportional to the erythema (microcirculation/ redness) of the skin. A higher a* value corresponds to higher erythema.
The b* value located on the blue-yellow axis in literature often describes sallowness.
In addition, the Individual Typography Angle (ITAo) is automatically calculated from L* (luminance) and b* (yellow chromatic). ITAo is an established tool to objectify skin colour for predicting the biological consequences of UV exposure on the skin (the lighter the skin, the higher the consequences) (Table 1) [18].
Results
In vitro: The average sGAGs concentration of OceanDerMX® products is 23.38 mg/mL (± 5.25) (Figure 1).
OceanDerMX® Balance & Brighten holds a relative total antioxidant capacity of 79% compared to the Trolox standard (Figure 2).
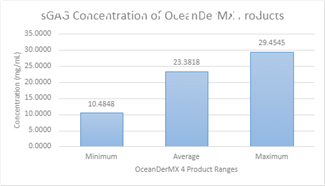
Figure 1. (In vitro) Average sGAGs quantity in OceanDerMX® products: 23.3818 mg/mL ± 5.25572
In vivo
Panel description and study follow-up: 27 healthy female Asian volunteers aged 18 and above completed the study and were included for statistical analysis of the parameters measured. No serious adverse events or adverse events were reported. The product is very well tolerated.
Skin lightness and brightness levels at D14: After 14 days of product application, there was a significant improvement in all parameters L* (p = 0.042), a* (p = 0.014), b* (p = 0.03) and ITAo (p = 0.002) tested, indicating that the product has a lightening and brightening effect (Figure 3, Table 3). The self-evaluation questionnaires support these results, indicating that 93% of participants felt they had a clearer complexion and 82% described their skin as more radiant on Day 14 (Table 4).
Table 2. The expected improvement of spectrophotometer® parameters
|
D14 |
D28 |
D56 |
P-value |
0.042035 |
0.00017 |
0.000103 |
Table 3. P-values showing statistical significance of the L* parameter
|
D14 |
D28 |
D56 |
Clearer complexion |
93% |
96% |
96% |
Moisturized |
89% |
96% |
92% |
Smooth |
93% |
92% |
100% |
Radiant |
82% |
89% |
89% |
Table 4. Table of percent (%) results from self-evaluation questionnaire
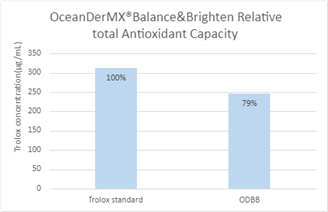
Figure 2. Relative total antioxidant capacity of OceanDerMX® Balance & Brighten compared against Trolox standard
Combined evaluation of skin brightness levels at D14, D28, and D56: After 28 days of product application, there was a significant improvement in the L* (p = 0), a* (p = 0), and ITAo (p = 0.002) parameters, indicating that the product has lightening and brightening effects (Figure 3-5, Table 3). The L* parameter increases between Day 14 and Day 56 by 1%, with there being a minimal difference in the L* parameter value between Day 14 and Day 28 (Figure 5). There is a significant decrease in the a* parameter over time (Figure 4). Compared to the baseline, after 14, 28, and 56 days, skin redness decreased by 3%, 9%, and 14% respectively (Figure 4).
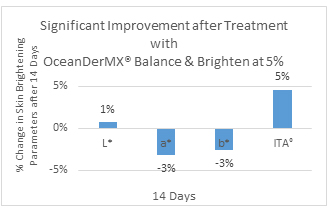
Figure 3. (In vivo) Significant improvement after treatment (14 days) with OceanDerMX® Balance & Brighten at 5%
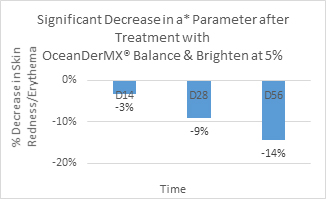
Figure 4. Significant improvement in a* parameter after treatment with OceanDerMX® Balance and Brighten at 5%
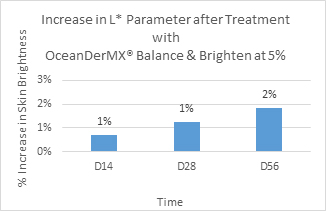
Figure 5. Significant improvement in L* parameter after treatment (14, 28, and 56 days) with OceanDerMX® Balance & Brighten at 5%
Clinical photographs
Baseline D14
D28 D56
Skin lightening efficacy of OceanDerMX® Balance & Brighten at 5%: Pigmentation parameters of the facial skin were measured on Day 14, Day 28 and Day 56 for 27 participants. Each timepoint was evaluated against the baseline of Day 0, showing a continual improvement in the whiteness and brightness of the skin across the duration of the study (Figure 3-5).
The improvement in skin brightness over time is supported by the self-evaluation questionnaires, showing an increase in participants perceiving they had a clearer complexion on Day 28 and Day 56 compared to Day 14 (Table 4). Similarly, there is an increase in participants perceiving their skin to be more radiant on Day 28 and Day 56 compared to Day 14 (Table 4).
Illustration of clinical efficacy: The clinical photographs show a reduction in pigmentation and/or brightening effects of OceanDerMX® Balance & Brighten at 5% after 14, 28 and 56 days (Figure 6). Photographs were taken using the Visia®-CR imaging system, using the S2 (standard 2: flat lighting) mode.
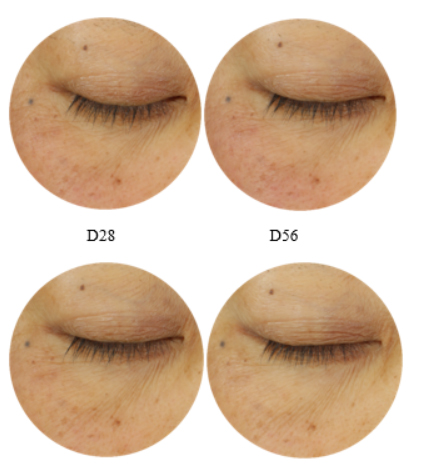
Figure 6. Clinical photographs of participant’s under-eye area using Visia®-CR skin imaging system on S2 (standard 2: flat lighting) mode
Overview of self-evaluation ratings: No adverse effects were recorded: the product is very well tolerated. 96% of the subjects had a positive overall appreciation of the product. 93% of the subjects would like to continue using the product. ~90% (+10%) of participants reported a clearer complexion with moisturised, radiant and smooth skin on days 14, 28 and 56, with the percentage of participants in agreeance with the questionnaire increasing throughout the study (Table 4).
Discussion
Skin is our largest organ and exists in a vast range of colours. This is due to the pigment known as melanin which is made in the deeper layers of the epidermis and expressed on the surface [7]. Perhaps melanin’s most crucial role is to serve as a physical barrier and protect us from environmental and physiological stressors such as UV rays [7]. In other words, the skin is a network of immunity, providing a unique defence mechanism against UV rays through its pigments [7].
Melanocytes are stimulated by UV rays and free radicals, from oxidants or inflammation, leading to the production of melanin. Thus, reducing melanin production by inhibiting tyrosinase through antioxidant and anti-inflammatory activity is a critical part of treating hyperpigmentation [8]. Existing research paints Centella asiatica (Gotu Kola) and Glycyrrhiza glabra (Liquorice root) as suitable tyrosinase inhibitors, through their ability to reduce melanin content in melanocytes by the downregulation of tyrosinase in B16F0 mouse melanoma cells [13,17].
OceanDerMX® Balance & Brighten, a proprietary blend of extracts from New Zealand, including native red seaweed (Sarcothalia circumcincta), native Mamaku/Black fern (Cyathea medullaris), Gotu Kola (Centella asiatica) and Liquorice root (Glycyrrhiza glabra), does have a lightening and brightening effect on the facial skin of the 27 participants in this study, as shown in the positive results for all pigmentation parameters, L*, a*, b*, and ITAo (Figure 3), corroborated by the decrease in clinical visibility of pigmentation (Figure 6). OceanDerMX® Balance & Brighten 5% led to an increase in brightness (L*), decrease in redness (a*), decrease in sallowness (b*), and increase in lightness (ITAo) of the skin within 14 days (Figure 3), demonstrating the lightening and brightening capabilities of this product. These results are sustainable as there was a continued decrease in redness (a*) between day 14 and day 56 (-11% difference) (Figure 4). Likewise, there was an increase in brightness (L*) from day 14 to day 56 (1% difference) (Figure 5).
Zolghadri and colleagues (2019) compiled the knowledge of natural as well as synthetic tyrosinase inhibitors biochemically characterised in the last four decades, reviewing their inhibitory effects [8]. Whilst the natural resource of tyrosinase inhibitors exist in a vast range, few are proven to be both safe and effective [8]. It is, therefore, recommended to examine the inhibitor’s safety and efficacy through both in vitro and in vivo means [8]. Our results are promising, with OceanDerMX® Balance & Brighten holding a relative total antioxidant capacity of 79% (Figure 2). The average sGAGs concentration of OceanDerMX® products is 23.38 mg/mL (± 5.25) (Figure 1), attributed to New Zealand native red seaweed (Sarcothalia circumcincta).
Tyrosinase inhibitors in the OceanDerMX® Balance & Brighten blend work together with New Zealand native red seaweed (Sarcothalia circumcincta) and Mamaku/Black fern (Cyathea medullaris) to deliver the desired results. These ingredients, through the method of extraction, are locked into the 3D-biopolymer fishnet structure of OceanDerMX® Balance & Brighten and do have a positive effect, increasing skin lightness, as shown in this study. Both New Zealand native red seaweed (Sarcothalia circumcincta) and Mamaku/Black fern (Cyathea medullaris) display excellent water holding capabilities to hydrate and tighten the skin through the presence of sulphated glycosaminoglycans (sGAGs) and Glucoronomannans respectively [10,14], likely leading to ~90% (+10%) of participants reporting a clearer complexion with moisturised, radiant and smooth skin on days 14, 28 and 56 (Table 4). The increase in the percentage of participants in agreeance with the questionnaire over the 56 days is supportive of the data which also follows a positive trend and attests to the functional mechanisms of the active compounds (Figure 3-5, Table 4).
OceanDerMX® Balance & Brighten at 5% concentration demonstrates both efficacious and safe results, with no reports of adverse events in our in vivo trial. The product was very well tolerated in the homogenous Asian population of this study. Our study demonstrates that topical application OceanDerMX® Balance & Brighten at 5% concentration can improve pigmentation, displaying significant lightening and brightening potential as a cosmetic ingredient from natural origins.
Through the synchronised modes of action resulting from the synergistic extraction process, New Zealand native red seaweed (Sarcothalia circumcincta) and Mamaku/Black fern (Cyathea medullaris) and Centella asiatica (Gotu Kola) and Glycyrrhiza glabra (Liquorice root) create a single multi-tasking ingredient. The product’s success may be attributed to the multiple ingredients working together to combat pigmentation and even out skin tone, giving the skin an overall more radiant and brighter appearance as present in all facets of analysis such as the improvement of all pigmentation parameters, clinical visibility, and self-evaluation by the participants.
Conclusion
The clinical study conducted as well as the in vitro test performed provide strong evidence for the efficacy of OceanDerMX® Balance & Brighten (at 5% in a cosmetic cream) for its lightening and brightening effects on the facial skin of 27 healthy female Asian volunteers starting as early as 14 days and continuing over 56 days. These sustainable results provide further scientific evidence into the role plant botanicals play in reducing photo-ageing pigmentation.
Author contribution
All authors have read and agreed to the published version of the manuscript and have contributed equally to its content.
Institutional review board statement
The clinical study was conducted according to the guidelines of the Declaration of Helsinki, OECD Good Laboratory Practices (GLP) and within the national guidelines of China.
Informed consent statement
Informed consent was acquired from all participants involved in this study.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
The authors wish to thank Bethany Park for her contribution in writing the paper.
References
- Awang DV (1998) Gotu kola: CPJRPC. CPJ. Canadian Pharmaceutical Journal 131: 42.
- Chandrika UG, Kumara PP, Gotu Kola (2015) (Centella asiatica): Nutritional Properties and Plausible Health Benefits. Advances in Food and Nutrition Research 76: 125-157.
- Khanom F, Kayahara H, Tadasa K (2000) Tyrosinase Inhibitory Activity of Bangladeshi Indigenous Medicinal Plants. Bioscience, Biotechnology, and Biochemistry 64: 1967-1969.
- Orhan IE, Atasu E, Senol FS, Ozturk N, Demirci B, et al. (2013) Comparative studies on Turkish and Indian Centella asiatica (L.) Urban (gotu kola) samples for their enzyme inhibitory and antioxidant effects and phytochemical characterization. Industrial Crops and Products 47: 316-322.
- Nerya O, Vaya J, Musa R, Izrael S, Ben-Arie R, et al. (2003) Glabrene and Isoliquiritigenin as Tyrosinase Inhibitors from Licorice Roots. Journal of Agricultural and Food Chemistry 51: 1201-1207. [Crossref]
- Vaibhav S, Lakshaman K (2012) Tyrosinase enzyme inhibitory activity of selected Indian herbs. Int J Res Pharm Biomed Sci 3: 977-982.
- Costin GE, Hearing VJ (2007) Human skin pigmentation: melanocytes modulate skin colour in response to stress. The FASEB Journal 21: 976-994.
- Zolghadri S, Bahrami A, Khan MTH, Munoz-Munoz J, Garcia-Molina F, et al. (2019) A comprehensive review on tyrosinase inhibitors. Journal of Enzyme Inhibition and Medicinal Chemistry 34: 279-309. [Crossref]
- Ferreira MS, Magalhaes MC, Oliveira R, Sousa-Lobo JM, Almeida IF (2021) Trends in the Use of Botanicals in Anti-Aging Cosmetics. Molecules 26: 3584. [Crossref]
- Shafie MH, Kamal ML, Zulkiflee FF, Hasan S, Uyop NH, et al. (2022) Application of Carrageenan extract from red seaweed (Rhodophyta) in cosmetic products: A review. Journal of the Indian Chemical Society 99: 100613.
- Ribeiro AS, Estanqueiro M, Oliveira MB, Sousa Lobo JM (2015) Main Benefits and Applicability of Plant Extracts in Skin Care Products. Cosmetics 2: 48-65.
- Gupta A, Naraniwal M, Kothari V (2012) Modern extraction methods for preparation of bioactive plant extracts. International journal of applied and natural sciences 1: 8-26.
- Kwon KJ, Bae S, Kim K, An IS, Ahn KJ, et al. (2014) Asiaticoside, a component of Centella asiatica, inhibits melanogenesis in B16F10 mouse melanoma. Molecular Medicine Reports 10: 503-507.
- Carnachan SM, Bell TJ, Hinkley SFR, Sims IM (2019) Polysaccharides from New Zealand Native Plants: A Review of Their Structure, Properties, and Potential Applications. Plants 8: 163. [Crossref]
- Jesumani V, Du H, Aslam M, Pei P, Huang N (2019) Potential Use of Seaweed Bioactive Compounds in Skincare-A Review. Marine Drugs 17: 688. [Crossref]
- Goh KKT, Matia-Merino L, Pinder DN, Saavedra C, Singh H (2011) Molecular characteristics of a novel water-soluble polysaccharide from the New Zealand black tree fern (Cyathea medullaris). Food Hydrocolloids 25: 286-292.
- Kang MH, Jang GY, Ji Y-J, Lee JH, Choi SJ, Hyun TK, et al. (2021) Antioxidant and Anti-Melanogenic Activities of Heat-Treated Licorice (Wongam, Glycyrrhiza glabra × G. uralensis) Extract. Current Issues in Molecular Biology 43: 1171-1187.
- Courage + Khazaka electronic GmbH. Skin-Colorimeter CL 400: Measurement Principle [Internet]. Köln, Germany: Courage + Khazaka electronic GmbH; [date unknown].






