Background: Total neoadjuvant therapy (TNT) represents a promising paradigm for patients with gastroesophageal (GE) and gastric (G) cancer, as approximately only half of these patients complete postoperative chemotherapy and/or chemoradiation therapy (CRT). TNT, consisting of neoadjuvant chemotherapy followed by CRT and surgery, may improve treatment delivery as compared to approaches including a postoperative chemotherapy component, but data are lacking regarding clinical outcomes of this approach.
Methods: We retrospectively analyzed patients who underwent locally advanced GE/G cancer resection after receiving TNT. TNT consisted of neoadjuvant FOLFOX or FLOT followed by CRT (GE 50.4 Gy/G 45 Gy) and surgery. Dose modifications occurred at the treating oncologist’s discretion. Our primary aim was to determine rates of TNT completion (defined as all 8 cycles of FLOT or FOLFOX, CRT, and resection). Secondary aims included treatment dose intensity, surgical outcomes, adverse effects, and healthcare utilization. This is the first study to explore TNT (including CRT) for GE/G cancer patients treated with FLOT and adds to growing evidence for TNT (including CRT) for those receiving FOLFOX.
Results: From 12/2015-4/2020, 61.2% (30/49) of patients completed TNT, including FLOT 68.8% (11/16) and FOLFOX 57.6% (19/33). The mean (±SD) age was 63.7 (±11.4) years, 85.7% White, and 73.5% male. Tumor locations included 42.9% GE, 44.9% G, and 12.2% overlapping sites. Overall, 24.5% of patients who received TNT had pathologic complete response (pCR). We found no significant difference in treatment intensity, R0 resection, pCR, adverse effects, or healthcare utilization between neoadjuvant FLOT versus FOLFOX.
Conclusion: In this cohort, more than 60% of patients with locally advanced G/GE cancer completed TNT, consisting of 8 cycles of FLOT or FOLFOX, CRT, and surgery with a median of 6.9 out of 8 cycles. The TNT approach warrants further evaluation in a larger, prospective study in patients with locally advanced GE/G cancer.
Worldwide, combined esophageal and gastric cancers (esophagogastric cancer) represent the third most common cancer overall and the second leading cause of cancer death [1,2]. Most patients present with lymph node involvement, which correlates with worse survival outcomes [3,4]. Surgical resection is the major curative treatment for esophagogastric cancers, but recurrence rates are high, likely due to occult metastatic disease [5-7]. The treatment of gastroesophageal and gastric (GE/G) cancers includes combinations of chemotherapy, radiation, and surgery in fit patients [8]. Historically, upfront surgery followed by chemotherapy and/or chemoradiation therapy (CRT) was the standard approach [9,10]. However, less than half of patients with esophagogastric cancer completed all post-operative chemotherapy and/or CRT [11,12]. As treatment options for locally advanced GE/G cancer evolved, multimodal approaches beyond initial curative resection largely replaced surgery followed by adjuvant chemotherapy and/or CRT [4,12-15].
Understanding the currently available evidence regarding multimodal approaches is critically important. The Southwest Oncology Group INT-0116 trial demonstrated improved overall survival and disease-free survival using adjuvant therapy—specifically CRT—compared to surgery alone [16]. Subsequently, several other studies have supported the use of postoperative chemotherapy and/or CRT in addition to surgery with improved outcomes [17-21]. This was followed by the MAGIC trial, which demonstrated a survival advantage for the use of perioperative combination chemotherapy (epirubicin, cisplatin, and 5-flurouracil [5-FU]) as compared to resection alone and prompted development of further pre- and post-operative multimodal approaches for localized G/GE cancers [22]. Subsequently, the CROSS trial demonstrated long-term overall survival benefits of neoadjuvant CRT in clinically resectable, locally advanced GE/G cancer and remains a standard approach across many cancer centers [8]. Despite widespread adoption, the 10-year results of CROSS are disappointing with only a 38% cure rate among those treated with neoadjuvant CRT where most failures were extraregional [23]. Most recently, the FLOT4 randomized controlled trial evaluated the impact of 4 cycles of FLOT (5FU, leucovorin, oxaliplatin, and docetaxel) prior to resection followed by 4 cycles after resection and observed a 15-month improved survival compared to patients that received perioperative ECF/ECX (epirubicin, cisplatin, 5FU / epirubicin, cisplatin, capecitabine). Importantly, the FLOT4 trial established a benchmark pCR rate of 16% in patients with locally advanced, resectable GE/G tumors [12]. However, less than half of patients completed all post-operative FLOT. Although current clinical guidelines recommend post- and peri-operative chemotherapy and/or neoadjuvant CRT for resectable GE/G tumors, purely preoperative (including image guided) approaches combining both systemic chemotherapy and CRT may improve therapy completion rates and elicit tumor down-staging before surgery [24,25].
We previously reported on the total neoadjuvant therapy (TNT) approach, consisting of modified FOLFIRINOX (5-FU, leucovorin, irinotecan, and oxaliplatin) followed by CRT and surgery, with 92% completing TNT and 28% experiencing a pathologic complete response (pCR) [26]. However, TNT clinical outcomes for patients receiving FLOT (5FU, leucovorin, oxaliplatin, docetaxel) [12] followed by CRT have not been reported and there are limited reports on patients receiving TNT with FOLFOX (5FU, leucovorin, oxaliplatin) followed by CRT and surgery. The current study describes our institutional experience with TNT in patients with locally advanced GE/G cancer, specifically evaluating two contemporary combination chemotherapy regimens of FLOT and FOLFOX followed by CRT. Our primary aim was to evaluate the rate of TNT completion and median number of chemotherapy doses received. Secondary aims included treatment intensity, surgical outcomes, adverse effects, and healthcare utilization. In comparison to our previously published modified FOLFIRINOX data [26], we hypothesized that patients receiving preoperative FLOT or FOLFOX chemotherapy as part of TNT would experience comparable completion rates, surgical outcomes, adverse effects, and healthcare utilization.
Study design
We conducted a retrospective analysis of consecutive patients with locally advanced GE/G cancers, who received their care at Massachusetts General Hospital (MGH) Cancer Center between December 2015 and April 2020. This study was approved by Dana-Farber/Harvard Cancer Center Institutional Review Board.
Patient cohort
Patients were considered eligible for the current study if they were ≥18 years old, had a known diagnosis of locally advanced GE/G cancer, had received at least 1 cycle of a prescribed FLOT or FOLFOX-based TNT, underwent resection, and received their care at MGH. A total of 49 patients met these criteria. Chemotherapy dose modification occurred at the discretion of the treating oncologist. Chemotherapy sensitizing agents with radiotherapy included standard regimens including carboplatin/paclitaxel, capecitabine, infusional 5-FU, and continued FOLFOX. Patients were treated with radiotherapy to a dose of 45 - 50.4 Gy in 28 fractions.
Data collection, definitions, and outcomes
We collected demographic information and disease-related variables (weight, cancer type, treatment, and toxicity) from the electronic health record. We defined complete TNT as 8 cycles (4 months) of FOLFOX (5-fluorouracil 2,800mg/m2, folinic acid 350 mg/m2, oxaliplatin 85mg/m2) or FLOT (5-fluorouracil 2,600mg/m2, oxaliplatin 85 mg/m2, docetaxel 50 mg/m2), followed by CRT (GE 50.4 Gy / G 45 Gy), and then surgical resection. Treatment intensity was defined as the percent of total prescribed complete neoadjuvant chemotherapy and radiation received prior to surgery. Adverse effects were captured as documented by the treating oncologists in progress notes. Time to resection was defined from date of the first cycle of chemotherapy to date of resection, and R0 resection was defined as removal of all residual macroscopic or microscopic disease. pCR was defined as the absence of residual invasive cancer on pathologic evaluation of the resected specimen and all sampled regional lymph nodes following completion of neoadjuvant systemic therapy and CRT. Otherwise, pathologic stage was defined according to American Joint Committee on Cancer guidelines (8th edition) [27].
Statistical analysis
We used descriptive statistics to estimate frequencies, means, and standard deviations. Additionally, we compared clinical outcomes between FLOT and FOLFOX groups using Wilcoxon rank-sum tests and Fisher’s exact tests as appropriate. A two-sided significance level of 0.05 was used for all comparisons.
We identified 49 patients with locally advanced GE/G cancer who were prescribed curative-intent TNT with either FLOT (n=16) or FOLFOX (n=33) followed by CRT and surgery. Overall, 61.2% (30/49) of patients completed all 8 cycles of TNT, including 68.8% FLOT and 57.6% FOLFOX (Figure 1). The median number of cycles of systemic chemotherapy received was 6.9 (out of a planned 8) and was similar between those receiving FLOT or FOLFOX. Patients were mostly White (42/49, 85.7%) and male (36/49, 73.5%) and had a mean age of 63.7 [±11.4] years. The histology of tumors was predominantly adenocarcinoma (96.8%), with primary tumor locations including 42.9% (n=21) GE, 44.9% (n=22) G, and 12.2% (n=6) overlapping sites (Table 1).
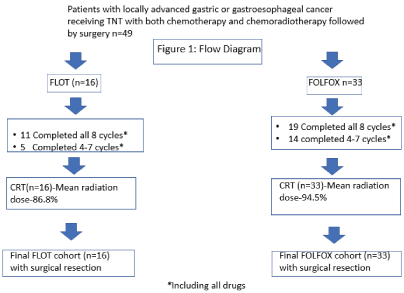
Figure 1. Flow Diagram
Table 1. Baseline patient and tumor characteristics
| |
Total
(n=49) |
FLOT
(n=16) |
FOLFOX
(n=33) |
P-value |
Age (mean years [±SD]) |
63.7 [±11.4] |
65.1 [±10.4] |
63.1 [±12.0] |
0.539 |
Sex (%)
Male
Female |
36 (73.5)
13 (26.5) |
13 (81.2)
3 (18.8) |
23 (69.7)
10 (30.3) |
0.502 |
Race (%)
White
Asian
Black
Other |
42 (85.7)
4 (8.2)
1 (2.0)
2 (4.1) |
15 (93.8)
1 (6.3)
0 (0.0)
0 (0.0) |
27 (81.8)
3 (9.1)
1 (3.0)
2 (6.1) |
0.874 |
Anthropometric (mean, [SD])
Baseline weight (kg)
Baseline body mass index (kg/m2) |
80.0 [±19.7]
29.0 [±9.0] |
77.8 [±18.9]
30.2 [±12.7] |
81 [±20.2]
28.4 [±6.7] |
0.581
0.592 |
Tumor location
Gastroesophageal
Gastric
Overlapping |
21 (42.9)
22 (44.9)
6 (12.2) |
4 (25.0)
9 (56.3)
3 (18.8) |
17 (51.5)
13 (39.4)
3 (9.1) |
0.230 |
Histology
Adenocarcinoma
Squamous cell carcinoma |
47 (95.9)
2 (4.1) |
16 (100.0)
0 (0.0) |
31 (93.9)
2 (6.1) |
0.449 |
Chemotherapy and chemoradiation
Neoadjuvant chemotherapy consisted of 32.6% (16/49) FLOT and 67.3% (33/49) FOLFOX. The percentages received of each chemotherapy agent prescribed were 86.5% 5-FU, 80.0% oxaliplatin, and 76.8% docetaxel for patients who received FLOT; and 75.5% 5-FU, and 81.7% oxaliplatin for patients who received FOLFOX (Table 2). The most common reasons for not completing all planned neoadjuvant FLOT or FOLFOX was toxicity as documented by oncologists. Nearly all patients completed prescribed combined chemoradiotherapy (86.8% FLOT vs. 94.5% FOLFOX) (Table 2) with radiosensitizing chemotherapies including capecitabine (n=18, 36.7%), carboplatin/paclitaxel (n=14, 28.6%), FOLFOX (n=6, 12.2%), and infusional 5-FU (n=7, 14.3%) (Table 2).
Table 2. Total neoadjuvant therapy received prior to surgery
| |
Total
(n=49) |
FLOT
(n=16) |
FOLFOX
(n=33) |
P-value |
Median number of systemic chemotherapy cycles received [±IQR]) |
6.9 [±1.7] |
7.2 [±1.4] |
6.8 [±1.8] |
0.369 |
Systemic chemotherapy received of prescribed (%, [±SD])
5FU
Oxaliplatin
Docetaxel
Total |
79.1 [±24.7]
81.1 [±26.3]
76.8 [±6.9]
79.1 [±24.6] |
86.5 [±23.0]
80.0 [±24.0]
76.8 [±6.9]
86.1 [±23.0] |
75.5 [±25.0]
81.7 [±27.7]
--
75.6 [±25.0] |
0.139
0.830
--
0.161 |
Chemoradiation type (%)
Capecitabine
Carboplatin/paclitaxel
FOLFOX
Infusional 5FU
None |
18 (36.7)
14 (28.6)
6 (12.2)
7 (14.3)
4 (8.2) |
5 (31.3)
9 (56.3)
0 (0.0)
0 (0.0)
2 (12.5) |
13 (39.4)
5 (15.2)
6 (18.2)
7 (21.2)
2 (6.1) |
0.007 |
Mean radiation dose received (cGy, [±SD]) |
4487.7 [±1226.3] |
4306.1 [±1694.4] |
4578.4 [±1226.3] |
0.556 |
Radiation received of prescribed (%, [±SD]) |
92.0 [±2.6] |
86.8 [±34.0] |
94.5 [±20.7] |
0.414 |
Surgery
Overall, we observed no difference in surgical outcomes between patients receiving neoadjuvant FLOT versus FOLFOX. The mean time from initiation of chemotherapy to surgical resection was 6.7 months, including 7.1 months for patients who received FLOT and 6.6 months for patients who received FOLFOX. Overall, the types of surgical procedures included left thoracoabdominal esophagogastrectomy (n=14, 28.6%), subtotal gastrectomy (n=13, 26.5%), Ivor Lewis esophagectomy (n=12, 24.5%), total gastrectomy (n=9, 18.4%), and 3-hole esophagectomy (n=1, 2%), without any differences between groups (Table 3). For patients receiving FLOT, 100.0% (16/16) had an R0 resection, 31.3% (5/16) pCR, and a mean post-surgical hospital length of stay of 14.1 days. For patients receiving FOLFOX, 97.0% (32/33) had a R0 resection, 21.2% (7/33) pCR, and an 8.7-day length of stay.
Table 3. Summary of surgical outcomes, adverse effects, and healthcare utilization
| |
Total
(n=49) |
FLOT
(n=16) |
FOLFOX
(n=33) |
P-value |
Time from chemotherapy start to resection (months) |
6.7 [±1.9] |
7.1 [±2.0] |
6.6 [±1.8] |
0.380 |
Surgical procedure (%)
LTA esophagogastrectomy
Subtotal gastrectomy
Ivor Lewis esophagectomy
Total gastrectomy
3-hole esophagectomy |
14 (28.6)
13 (26.5)
12 (24.5)
9 (18.4)
1 (2.0 |
8 (50.0)
2 (12.5)
4 (25.0)
2 (12.5)
0 (0.0) |
6 (18.2)
7 (21.2)
8 (24.2)
7 (21.2)
1 (3.0) |
0.163 |
R0 resection (%, [±SD]) |
98.0 [±14.3] |
100.0 [±0] |
97.0 [±14.3] |
0.3248 |
Pathologic complete response (%) |
12 (24.5) |
5 (31.3) |
7 (21.2) |
0.492 |
Lymph node positivity (%) |
0.3 [±0.4] |
0.4 [±0.5] |
0.2 [±0.4] |
0.235 |
Post-surgery hospital length of stay (days) |
10.4 [±11.3] |
14.1 [±17.1] |
8.7 [±6.7] |
0.1183 |
Adverse effects (baseline to resection, %)
Weight loss (kg, [±SD])
Neuropathy (%)
Neutropenic fever (%)
Onycholysis (%) |
-4.5 [±8.4]
36 (73.5)
5 (10.2)
6 (12.2) |
-4.2 [±6.5]
12 (75.0)
2 (12.5)
4 (25.0) |
-4.6 [±9.2]
24 (72.7)
3 (9.1)
2 (6.1) |
0.851
0.577
0.532
0.080 |
Healthcare utilization (mean, [±SD])
Hydrations
Emergency department visits
Unplanned hospitalizations |
1.9 [±3.5]
0.3 [±0.7]
0.8 [±1.3] |
3.5 [±4.4]
0.1 [±0.3]
1.0 [±1.5] |
1.2 [±2.7]
0.4 [±0.9]
0.6 [±1.1] |
0.065
0.091
0.408 |
Adverse effects and healthcare utilization
We found no differences in adverse effects or healthcare utilization from time of chemotherapy initiation to surgical resection. On average, patients who received FLOT experienced a 4.2 kg weight loss, 75% of patients experienced any grade neuropathy, 12.5% had a neutropenic fever, and 25% had onycholysis. Patients who received FOLFOX experienced an average 4.6 kg weight loss, 72.7% of patients experienced any grade neuropathy, 9.1% had a neutropenic fever, and 6.1% had onycholysis. Grades of each adverse effect were not available in all progress notes and therefore could not be reported. We observed no differences in healthcare utilization between groups. Patients who received FLOT required an average of 3.5 hydrations, 0.1 ED visits, and 1.0 hospitalizations during neoadjuvant chemotherapy. In contrast, patients who received FOLFOX needed 1.2 hydrations, 0.4 ED visits, and 0.6 unplanned hospitalizations.
To our knowledge, this is the first study to report FLOT-based systemic chemotherapy followed by chemoradiotherapy TNT outcomes in patients with locally advanced GE/G cancers, as well as adding to information on FOLFOX based TNT followed by chemoradiotherapy in patients with locally advanced GE/G cancers. We found that over 60% of patients completed TNT, including 8 cycles of neoadjuvant chemotherapy, CRT, and surgery. Specifically, 68.8% of patients completed prescribed FLOT compared to 57.6% FOLFOX, and nearly all patients completed the prescribed CRT. The median number of cycles of systemic chemotherapy received was 6.9 out of 8 planned and was similar between the two regimens including the percentage of the total dose of each chemotherapy agent received (between 75.5 and 86.5%) (Table 2). At the time of surgery, nearly all patients achieved an R0 resection and approximately a quarter attained a pCR (Table 3). We also found reasonable adverse event profiles, indicating that these modalities are also tolerable. We did not observe any statistically significant differences in surgical outcomes, adverse effects, or healthcare utilization (Table 3). Collectively, these data suggest that both FLOT- and FOLFOX-based TNT followed by CRT approaches demonstrate promising completion rates with comparable outcomes in terms of the above clinical parameters.
We have previously reported our experience in using modified FOLFIRINOX as part of TNT in a similar patient population (albeit with specific eligibility criteria on a clinical trial) [26]. In this prior study, nearly all patients (92%, 23/25) completed all 8 cycles of FOLFIRINOX followed by CRT and surgery. Moreover, 80% (20/25) underwent surgical resection, including 95% R0 resection and 28% pCR with acceptable rates of adverse effects. Taken together, our results in combination with other recently reported, support the ongoing continued evaluation of TNT in patients with locally advanced GE/G cancer using either FLOT or FOLFOX [28-30]. Given that rates of pCR are correlated with recurrence-free survival outcomes, the sum of our experience with FOLFIRINOX, FLOT, and FOLFOX in combination with those from recently reported studies suggests an early indicator that the TNT approach may be effective [25,26,28,31]. However, studies have yet to conclusively determine if survival outcomes can be improved with increased treatment intensity by delivering all chemotherapy prior to CRT and surgery.
It is important to place these preliminary results in context with modern perioperative chemotherapy. Despite many advances in multimodal therapy approaches for GE/G cancers, including MAGIC [22] FLOT [4,12] CROSS [8,23,32] and ACCORD [33] novel treatment paradigms are needed to improve clinical outcomes. Al-Batran and colleagues demonstrated that 4 cycles of FLOT prior to resection followed by 4 cycles after resection improved survival in patients with resectable GE/G cancer [12]. The FLOT4 randomized phase III trial established a benchmark pCR rate of 16% in patients with locally advanced, resectable tumors that ultimately led to a 15-month survival improvement compared to the perioperative ECF regimen.
Despite these multimodal approaches leading to encouraging survival outcomes, the risk of poor clinical outcomes in high-risk locally advanced patients remains unacceptably high [12,22,23,25,32-37]. This finding appears, at least in part, to be due to a significant percentage of patients who did not receive all of the planned chemotherapy postoperatively. The difficulty of delivering postoperative treatment is indicated by the results of a large prospective observational study, where only 13.6% of patients completed all post-operative FLOT, indicating the difficulty in delivering full doses of postoperative chemotherapy [37]. The importance of receiving all planned chemotherapy is suggested by a recent analysis indicating a survival advantage for patients who received postoperative chemotherapy [38]. The TNT approach, whereby patients receive chemotherapy and CRT before surgery, represents a promising alternative treatment strategy to mitigate the risks of recurrence [25,26,29,30,32]. Additionally, encouraging results from the phase II randomized study evaluating the use of PET response to guide treatment decisions during CRT after the initial induction therapy (FOLFOX or Carboplatin plus Paclitaxel chemotherapy) followed by a planned surgical resection. suggest a PET response to neoadjuvant FOLFOX improves pCR and survival supports the TNT approach to treating GE cancers [25]. Clearly, phase III trials are required to establish whether TNT followed by CRT and surgery improves survival as compared to other approaches including CRT alone, perioperative chemotherapy, or adjuvant treatment. A number of ongoing phase III trials are addressing various aspects of this [39-42]. In addition, in attempts to further improve these approaches, ongoing trials are evaluating combinations of chemotherapy with immune checkpoint inhibitors or chemotherapy combined with agents targeting HER2 (such as trastuzumab) for patients with HER2+ gastroesophageal cancers, both based on improved survival with these approaches in the metastatic disease setting [43,44].
Notable limitations of this study include its retrospective design, the small size of the study population, and limited generalizability since it was conducted at a single academic institution. We also recognize selection bias, as we focused our analysis on those patients who completed at least a portion of their TNT followed by surgery and lack outcomes of patients who were not candidates for the TNT approach. Furthermore, since we relied on documentation of adverse events in the medical record rather than capturing patient-reported outcomes, evaluation of adverse treatment effects is likely understated. We also limited our evaluation from the time of initiation of chemotherapy to surgical resection and therefore lacked survival outcomes. Future directions will include capturing data on all patients with G/GE cancer considered for TNT and presenting survival data of the current cohort.
Current literature lacks evidence regarding safety and efficacy for total neoadjuvant FLOT, and limited reports of total neoadjuvant FOLFOX, each followed by CRT before surgery for GE cancers. Despite previous studies supporting higher completion rates for neoadjuvant therapy as compared to perioperative therapy, the current work represents the first to explore TNT completion rates, pCR rates, and adverse effects for total neoadjuvant FLOT with CRT and adds to the growing evidence regarding total neoadjuvant FOLFOX, with CRT in this patient population. This analysis suggests that neoadjuvant chemotherapy followed by CRT may be a promising approach. Findings from this study support the need for ongoing and future clinical trials investigating the safety and efficacy of various TNT regimens, including determining if higher pCR will translate into a meaningful difference in disease-free survival and/or overall survival [31]. Future directions should also include a prospective comparison amongst contemporary neoadjuvant chemotherapy combinations (FOFIRINOX, FLOT, FOLFOX), including surgical, pathologic, patient-reported outcomes, healthcare utilization, and survival outcomes. We hope our exploratory TNT experience can further inform future interventions in patients with locally advanced GE/G cancer.
We would like to recognize the incredible strength and determination of our patients and their caregivers as well as the support of our multidisciplinary colleagues.
Eric J. Roeland – is currently, or has recently been (last 24 months) serving as a consultant for Mitobridge Inc., Asahi Kasei Pharmaceuticals, DRG Consulting, Napo Pharmaceuticals, American Imaging Management, Immuneering Corporation, Prime Oncology; additionally, he has served on recent advisory boards for Heron Pharmaceuticals, Vector Oncology; and has served as a member on data safety monitoring boards for Oragenics, Inc, Galera Pharmaceuticals, Enzychem Lifesciences Pharmaceutical Company.
Samuel J. Klempner – consultant/advisory role for Astellas, Sanofi-Aventis, Merck, BMS, Daiichi-Sankyo, Eli Lilly, AstraZeneca, and Pieris Oncology. SJK declares stock ownership in Turning Point Therapeutics, Inc.
Avinash Kambadakone- Research Grant- Philips Healthcare, GE Healthcare, PanCAN.
Aparna Parikh Foundation Medicine, Natera, Checkmate Pharmaceuticals, Eli Lilly, Pfizer, and Roche; and other from Puretech, PMV Pharma, BMS, Novartis, Plexxicon, Takeda, Macrogenics, C2I genomics.
David Ryan- MPM; other support from Acworth Pharmaceuticals; personal fees from Iteos, Uptodate, McGraw Hill, and Boehringer Ingelheim; non-financial support from Exact Sciences; and grants and personal fees from SU2C during the conduct of the study; personal fees and other support from MPM; other support from Acworth Pharmaceuticals, Exact Sciences; personal fees from Iteos, Uptodate, McGraw Hill, and Boehringer Ingelheim, and grants and personal fees from SU2C outside the submitted work.
Ryan Corcoran reports personal fees from Abbvie, Pfizer, Astex Pharmaceuticals, Chugai, Elicio, Fog Pharma, Guardant Health, Ipsen, Mirati Therapeutics, Natera, Navire, Qiagen, Roivant, Shionogi, Tango Therapeutics, Taiho, and Zikani Therapeutics; grants and personal fees from Asana Biosciences and AstraZeneca; personal fees and other from Avidity Biosciences, C4 Therapeutics, Kinnate Biopharma, nRichDx, Remix Therapeutics, and Revolution Medicines; and other from Erasca outside the submitted work.
Lipika Goyal reports receiving research funding (to institution) from Agios, Adaptimmune, Bayer, Eisai, Merck, Macrogenics, Genentech, Novartis, Incyte, Eli Lilly, Loxo Oncology, Relay Therapeutics, QED, Taiho Oncology, Leap Therapeutics, Bristol Meyers Squibb, and Nucana; scientific advisory committee (to self) from Agios Pharmaceuticals Inc, Alentis Therapeutics AG, H3Biomedicine, Incyte Corporation, QED Therapeutics, Sirtex Medical Ltd, and Taiho Oncology Inc.; consulting (to self) from Agios Pharmaceuticals Inc, Alentis Therapeutics, Genentech, Exelixis, Incyte Corporation, QED Therapeutics, Sirtex Medical Ltd, and Taiho Oncology Inc.; and DSMC (to self) from AstraZeneca.
Michael Lanuti – Consultant Astrazenaca.
T.S. Hong- Merck, Novocure, and Synthetic Biologics outside the submitted work.
Consultant/Advisory board – Bayer.
No disclosures were reported by the other authors.
- American Cancer Society (2021) Cancer Facts & Figures. Atlanta: American Cancer Society.
- Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, et al. (2020) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71: 209-249.
- Abdalla EK, Pisters PW (2004) Staging and preoperative evaluation of upper gastrointestinal malignancies. Seminars in oncology 31: 513-529. [Crossref]
- Ajani JA, D'Amico TA, Almhanna K (2016) Gastric Cancer, Version 3.2016, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network 14: 1286-1312.
- Cardoso R, Coburn N, Seevaratnam R (2012) A systematic review and meta-analysis of the utility of EUS for preoperative staging for gastric cancer. Gastric Cancer 15: 19-26. [Crossref]
- Spolverato G, Ejaz A, Kim Y (2015) Use of endoscopic ultrasound in the preoperative staging of gastric cancer: a multi-institutional study of the US gastric cancer collaborative. Journal of the American College of Surgeons 220: 48-56.
- Kutup A, Yekebas EF, Izbicki JR (2010) Current diagnosis and future impact of micrometastases for therapeutic strategies in adenocarcinoma of the esophagus, gastric cardia, and upper gastric third. Recent Results Cancer Res 182: 115-125.
- Shapiro J, Van Lanschot JJB, Hulshof MC (2015) Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. The lancet oncology 16: 1090-1098.
- Lerut T, Coosemans W, Decker G, De Leyn P, Moons J, et al. (2006) Diagnosis and therapy in advanced cancer of the esophagus and the gastroesophageal junction. Curr Opin Gastroenterol 22: 437-41. [Crossref]
- Pericay C, Macías-Declara I, Arrazubi V, Vilà L, Marín M (2019) Treatment in esophagogastric junction cancer: Past, present and future. Cir Esp (Engl Ed) 97: 459-464.
- Hughes BG, Yip D, Chao M, Gibbs P, Carroll S, et al. (2004) Audit of postoperative chemoradiotherapy as adjuvant therapy for resected gastroesophageal adenocarcinoma: an Australian multicentre experience. ANZ J Surg 74: 951-956.
- Al-Batran SE, Homann N, Pauligk C (2019) Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4). The Lancet 393: 1948-1957.
- Ajani JA, D’Amico TA, Bentrem DJ (2019) Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network 17: 855-883.
- Smyth E, Verheij M, Allum W (2016) Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology 27: 38-49.
- Lordick F, Mariette C, Haustermans K (2016) Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology 27: 50-57. [Crossref]
- Macdonald JS, Smalley SR, Benedetti J (2001) Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. New England Journal of Medicine 345: 725-730.
- Hundahl SA, Macdonald JS, Benedetti J (2002) Surgical treatment variation in a prospective, randomized trial of chemoradiotherapy in gastric cancer: the effect of undertreatment. Annals of Surgical Oncology 9: 278-286.
- Sakuramoto S, Sasako M, Yamaguchi T (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. New England Journal of Medicine 357: 1810-1820.
- Sasako M, Sakuramoto S, Katai H (2011) Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 29: 4387-4393. [Crossref]
- Noh SH, Park SR, Yang HK (2014) Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. The Lancet Oncology 15: 1389-1396.
- Lee J, Lim DH, Kim S (2012) Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin oncol 30: 268-273.
- Cunningham D, Allum WH, Stenning SP (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. New England Journal of Medicine 355: 11-20.
- Eyck BM, van Lanschot JJB, Hulshof M (2004) Ten-Year Outcome of Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: The Randomized Controlled CROSS Trial. J Clin Oncol 39: 1995-2004.
- Chapin WJ, Massa RC, Eads JR (2021) Evolving standards of care for neoadjuvant and adjuvant therapy in esophageal, gastroesophageal junction, and gastric cncer. Clin Adv Hematol Oncol 19: 784-793. [Crossref]
- Carr RA, Hsu M, Harrington CA (2021) Induction FOLFOX and PET-Directed Chemoradiation For Locally Advanced Esophageal Adenocarcinoma. Ann Surg 13.
- Wo JYL, Clark JW, Allen JN (2019) A pilot study of neoadjuvant FOLFIRINOX followed by chemoradiation for gastric and gastroesophageal cancer: Preliminary results. American Society of Clinical Oncology 2019: 4057.
- Solsky I, Palis B, Langdon-Embry M (2017) Validation of the 8th Edition of the AJCC TNM Staging System for Gastric Cancer using the National Cancer Database. Ann Surg Oncol 24: 3683-3691.
- Villanueva L, Anabalon J, Butte JM, Salman P, Panay S, et al. (2021) Total neoadjuvant chemotherapy with FLOT scheme in resectable adenocarcinoma of the gastro-oesophageal junction or gastric adenocarcinoma: impact on pathological complete response and safety. Ecancermedicalscience 15: 1168. [Crossref]
- Induction FLOT With CROSS CRT for Esophageal Cancer. Clinicaltrials NCT04028167.
- Preoperative Chemo and Chemoradiotherapy for Adenocarcinoma of the Stomach and Gastroesophageal Junction (GEJ). Clinicaltrials NCT00525785.
- Li Z, Shan F, Wang Y (2018) Correlation of pathological complete response with survival after neoadjuvant chemotherapy in gastric or gastroesophageal junction cancer treated with radical surgery: A meta-analysis. PLoS One 13: e0189294.
- van Hagen, Hulshof MC, van Lanschot JJ (2012) Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366: 2074-2084. [Crossref]/
- Boige V, Pignon J, Saint-Aubert B (2007) Final results of a randomized trial comparing preoperative 5-fluorouracil (F)/cisplatin (P) to surgery alone in adenocarcinoma of stomach and lower esophagus (ASLE): FNLCC ACCORD07-FFCD 9703 trial. Journal of Clinical oncology 25: 4510-4510.
- D'Angelica M, Gonen M, Brennan MF (2004) Patterns of initial recurrence in completely resected gastric adenocarcinoma. Annals of surgery 240: 808.
- van der Woude SO, Hulshof M, van Laarhoven H (2016) CROSS and beyond: a clinical perspective on the results of the randomized ChemoRadiotherapy for Oesophageal cancer followed by Surgery Study. Chinese clinical oncology 5:13.
- Tian S, Jiang R, Madden NA (2020) Survival outcomes in patients with gastric and gastroesophageal junction adenocarcinomas treated with perioperative chemotherapy with or without preoperative radiotherapy. Cancer 126: 37-45. [Crossref]
- Giommoni E (2021) Results of the observational prospective RealFLOT study. BMC Cancer 21: 1086.
- Rahman S, Thomas B, Maynard N (2021) On behalf of the NOGCA project team and AUGIS, Impact of postoperative chemotherapy on survival for oesophagogastric adenocarcinoma after preoperative chemotherapy and surgery. British Journal of Surgery znab427.
- Sah BK (2021) Dragon III-Phase 3: Neoadjuvant Chemotherapy (FLOT Versus SOX) for Gastric Cancer. ClinicalTrials NCT04384601.
- Hofheinz R (2020) Neoadjuvant RCT Versus CT for Patients with Locally Advanced, Potentially Resectable Adenocarcinoma of the GEJ. ClinicalTrials NCT04375605.
- Hoeppner J, Lordick F, Brunner T (2016) ESOPEC: prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer 16: 503. [Crossref]
- Buduhan G (2021) Preoperative Chemotherapy vs. Chemoradiation in Esophageal / GEJ Adenocarcinoma (POWERRANGER). ClinicalTrials NCT01404156.
- Janjigian YY, Shitara K, Moehler M (2021) First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 3: 27-40.
- Bang YJ, Van Cutsem E, Feyereislova A (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376: 687-97.
Editorial Information
Editor-in-Chief
Dung-Fang Lee
The University of Texas
Article Type
Research Article
Publication History
Received: February 02, 2022
Accepted: February 15, 2022
Published: February 27, 2022
Copyright
©2022 Eric J Roeland, Katie Kanter, Samuel J Klempner, Ryan D Nipp, Avinash Kambadakone, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Jeffrey W. Clark (2022) Exploring FLOT- and FOLFOX-Based total neoadjuvant therapy for patients with locally advanced gastroesophageal cancers. Cancer Rep Rev 6: DOI: 10.15761/CRR.1000238

