External medial maxillectomy is a common surgical approach for the management of para-nasal sinusoidal tumor, however tumor extension to nasopharynx is an extreme challenge to maxillofacial surgeon in planning surgical resection with adequate oncological control of the disease. This article will report a case of extensive adenocystic carcinoma of nasal cavity involving maxilla sinus with extension to ethmoid sinus superiorly and nasopharynx posteriorly. Patient had seen by few ENT surgeons for the management of severe nasal obstruction for more than 6 months without the consideration of neoplasm. The clinical diagnosis including histology, imaging workup in conjunction with 3D skull model printing to formulate the surgical resection & reconstruction plan will be well discussed. The operative technique of this extended external medial maxillectomy for enbloc resection of this tumor with ethmoidectomy superiorly and nasopharyngectomy posteriorly will be described in detail.
Adenoid cystic carcinoma (ACC) is an uncommon malignant tumor, it accounts for 2 and 3% of all upper aero-digestive tract malignancy with the incidence of 1% of all malignant tumors of the oral cavity and maxillofacial region [1,2]. ACC is the second most common malignancy in sinonasal tract cancer (nasal cavity, paranasal sinuses, and nasopharynx) [3]. It is most prevalent in minor salivary glands presents as small basaloid cells histology in solid, cribriform and trabecular pattern. Nasal obstruction symptoms had shown to be the commonest presentation in a cohort of eight-six cases of Sinonasal Tract Adenoid Cystic Carcinoma, followed by epistaxis 27%, auditory symptoms, nerve symptoms, nasal discharge and/or visual symptoms presented for a mean 18.2 months [4]. Herein, we report a case of ACC with nasal obstruction and para-alar swelling for more than 6 months and had seen few ENT surgeons with mismanagement as radiofrequency turbinate reduction. Finally, patient was referred to our specialist clinic for diagnosis of maxilla sinus/nasal cavity ACC and submitted for subtotal maxillectomy and nasopharyngectomy via external medial maxillectomy approach.
In January 2018, a 50-year-old female was referred to us for specialist surgical consultation of right para-alar swelling and large radiolucency at upper right incisors subapical region (Figure 1). She had suffered from more than 6 months nasal obstruction and had consulted for few ENT specialists for her obstruction symptom and had received a course of radiofrequency turbine reduction without any improvement. Clinical examination revealed a firm soft tissue mass at right para-alar region that obliterate the nasolabial groove and the ipsilateral inferior turbinate was displaced medially causing obstruction of the nostril aperture. The patient underwent biopsy via sub-labial incision to soft tissue mass eroding the piriform under local anaesthesia. Histology favour malignant salivary gland neoplasm with the differential diagnosis Adenocystic carcinoma, basal cell adenocarcinoma and pleomorphic carcinoma. Further CT and MRI confirmed tumor extension superiorly to the ethmoid sinuses without orbital content involvement and posteriorly to the nasopharynx (Figure 2 and 3). T2 fluid signal in right frontal, ethmoid and sphenoid sinuses which is consistent with sinusitis due to obstructive effect of the tumor. PET scan also showed no metastatic lesion. The patient underwent subtotal hemi maxillectomy in conjunction ethmoidectomy and nasopharyngectomy with preservation of orbital content via this extented external medial maxillectomy approach. Immediate free vascularized fibular graft to reconstruct the hard and soft tissue defect. For this extensive tumor involvement of mid-facial skeleton, pre-operative 3D printing of skull model was fabricated for resection planning particularly close to orbital apex and skull base region (Figure 4), additionaly it serves as a guide for three-dimensional reconstruction. Final histology confirmed Grade 2 ACC which is disposed in cribriform and tubular structures containing some mucinous materials in lumen. Basal cells were highlighted by immunostaining. There are features of perineural spread. All the resection margins were clear. After surgery, the patient had taken courses of adjuvant chemo-radiation therapy. Follow-up 17 months from initial diagnosis, she was still alive without any evidence of local recurrence and distance metastasis.
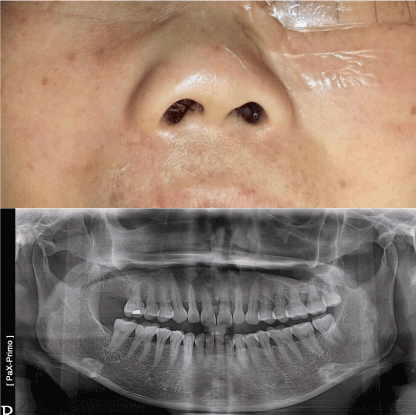
Figure 1. Right para-alar swelling and panoramic x-ray showed large radiolucent eroding lesion of the nasal floor at super-apical region of upper incisors
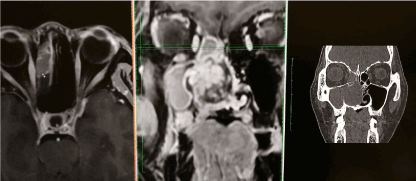
Figure 2. Multiplanar CT /MRI coordination to show the superior extension of the tumor to the ethmoid sinus
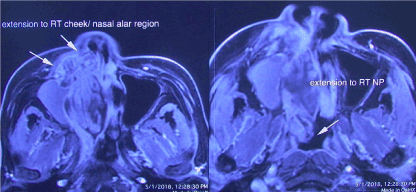
Figure 3. Axial MRI showed the anterior tumor extension to the alar cheek and fully obstructing the nasal cavity medially. Posteriorly the tumor was extent to ipslateral nasopharynx
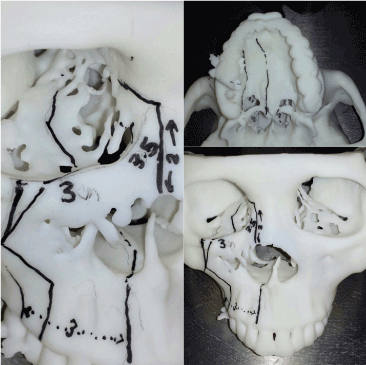
Figure 4. 3D skull model printing with precise measurements pre-operatively for surgical planning
Operative technique
The upper cheek flap approach or modified Weber-Fergusson incision has long been described as the standard surgical approach for maxilla tumor resection which is good for exposure of entire lateral aspect of the maxilla and the periorbital structures [5]. Moreover, tumor resection involving lateral nasal wall, medial wall of the maxillary sinus and the adjacent ethmoid cells was well described as external medial maxillectomy [6,7]. In this case, the tumor was extended posteriorly to nasopharynx, therefore nasopharyngectomy will perform as posterior margin control. Not until early 80’s, sophisticated surgical access including infratemporal fossa, anterolateral disassembly and maxillary and mandibular swing approaches were reported, the tumor in nasopharynx had traditionally been accepted to be inapproachable and non-resectable [8-12]. According to the MRI report, the frontal sinus opacity was only fluid signal therefore we could spare the frontal sinus as the superior most resection. Incorporate the boundaries of external medial maxillectomy for ethmoidectomy as medial half of orbit superiorly and the dental alveolus inferiorly due to the tumor invasion into maxilla sinus floor that will be categorized as Class 3a Subtotal maxillectomy (Brown’s classification) [13].The tumor was resected enbloc and hinged distally to gain full exposure of nasopharynx for nasopharyngectomy as posterior margin control. A stepwise approach of all the salient steps to illustrate this extended external medial maxillectomy case as followings:
Soft tissue dissection key steps
- Extended Weber-Fergusson incision with infraorbital incision and lip split incision (Figure 5) to expose the tumor abuting on the anterior maxilla wall and superiorly to lateral nasal bone for the medial orbital wall resection.
- Periorbital dissection with identification of medial canthal ligament, superior dissection is carried medially to retract orbital content. Meticulous dissection of the lacrimal sac and traochlea. Lateral dissection along the inferior orbital rim and infraorbital nerve foramen below.
- The medial canthal tendon is transacted and tagged for repair at the skin redrapping and the lacrimal duct also cut and could be consider re-cannulate at the end of procedure.
- Anterior and posterior ethmoid arteries will be encountered respectively and should be well cauterized using bipolar electrosurgery for further posterior subpeiosteal dissection for medial orbital wall resection. Martins et al has suggested the rule of seven in microsurgical anatomy of the orbit, the anterior ethmoidal vessel emerging from the canal is on average 21mm from the medial orbital edge, the posterior ethmoidal vessel is immediate 14mm posterior so as the optic canal is on average 7mm further posterior to posterior ethmoid canal (Figure 6) [14].
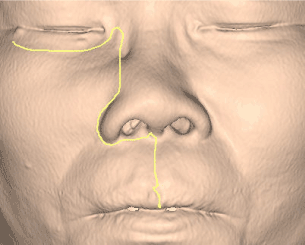
Figure 5. Weber-Fergusson incision with infraorbital incision and lip split incision
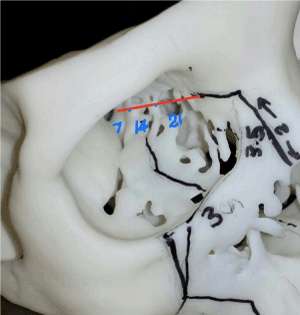
Figure 6. Completed osteotomized subtotal maxillectomy specimen before resection
Osteotomy key steps (guided by pre-operative 3D skull printing)
- First bone cut - forceps extraction of the ipsilateral central incisor to provide osteotomy access. Reciprocating sawing through the dental alveolus and full thickness osteotomy through the nasal floor and palate transorally with the preservation of the soft palate tissue aporn posteriorly.
- Second bone cut – mid-orbital rim osteotomy cut involving the medial orbital floor and infra-orbital nerve and the extension of cut towards laterally preserving the zygomatic prominence. It runs obliquely to the posterior maxilla wall just abuting to pterygoid maxillary fissure. Sawing the posterior wall of maxilla may lacerate the internal maxillary artery and could lead to devastating bleeding.
- Third bone cut – It made just inferior to fronto-ethmoidal suture line through the medial orbit. Stay inferior to this landmark will help avoid a cerebrospinal fluid leak. The orbital contents are retracted laterally during this osteotomy cut. The posterior orbital osteotmy will be finished by a thin osteotome tapping it continuous with the posterior orbital floor bone cut (Figure 7). Gentle retraction of the orbital content during bony wall osteotomy is essential to avoid ocular complications.
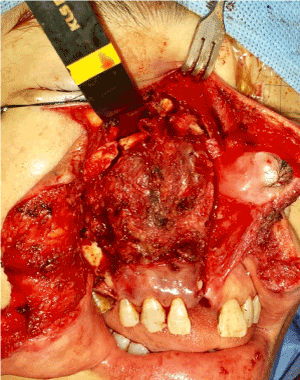
Figure 7. Medial orbital wall resection – sequential relationship of anterior/posterior ethmoid foramen and orbital apex
Maxillectomy & nasopharyngectomy
- Heavy curved pterygoid osteotome was passed through the maxilla tuberosity as pterygoid disjunction to complete the lateral posterior maxillary cut to mobilize the tumor mass enbloc within the maxillectomy specimen. Monopolar diathermy and heavy scissors dissection aim to complete the soft tissue resection along the bone cut posterior-medially. The high-risk sites are the posterior maxilla and posterior nasal aperture resection that could result in torrential bleeding. Cautious double ligation of the the descending palatine artery and keep the pterygoid venous plexus intact are the upmost important steps to provide a bloodless surgical field for clear visualization of the nasopharynx in this case.
- Once the hard and soft tissue resection complete, the maxillectomy specimen was swung laterally, the nasopharynx had already been well visualized with double magnification and headlight to assess the tumor margin (Figure 8). Dental local anaesthesia with adrenaline injection helps haemostasis before diathermy resection in close vicinity to fossa rose muller. When we use diathermy to peel off the mucosal margin, cautious in mind the internal carotid artery running across the nasopharyngeal region.
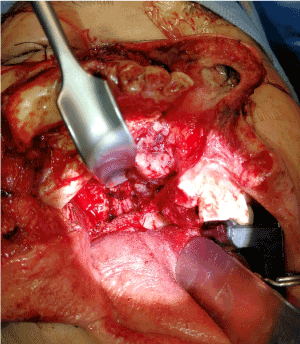
Figure 8. Maxillectomy specimen was swung laterally, so the nasopharynx was well visualized by illumination at the tip of retractor
Finally, the three-dimensional resection defect was reconstructed with a free vascular fibular graft to obturate the oral defect and support of the facial complex (Figure 9).
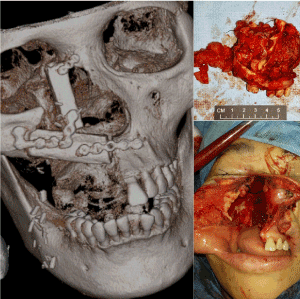
Figure 9. The oral orbitomaxillary composite resection defect was successfully reconstructed with free vascularized fibular graft
The 5-year overall survival and disease free-specific survival was 62% and 67% respectively reported in a metanalysis study of ACC of nasal cavity and paranasal sinuses. The local recurrence rate was 36.6% and the regional recurrence rate was 7%. Distance metastasis most commonly presented in the lung. In the international cohort, positive margins and ACC of the sphenoid or ethmoid sinuses were significantly predictors of outcome [15]. Therefore, adequate margin clearance is primarily important to treat this disease particularly for this patient. The most frequent presenting symptoms is nasal obstruction in primary sinonasal tract and nasopharyngeal adenoid cystic carcinoma, unfortunately this patient was managed excessively delayed even after few otolarynogologists’ consultation and mal- treated by radiofrequency reduction of her inferior turbinate [4]. This patient finally was referred by her general dentist because of the large radiolucency seen in the panoramic x-ray with toothache at upper incisors region. Therefore, both medical and dental practitioner should hold a high index of suspicion during consultation for those patients presenting with persistent regional signs and symptoms in oral maxillofacial area. Either CT and MRI studies serve well to delineate the tumor extension, whilst MRI could well differentiate the hyperdense signal from tumor or fluid occupancy that help us to decide the extend of resection. Technological advancement helps both the surgeon and oncologist in pre- and post-operative planning of head and neck tumor management protocol.
The application of 3D imaging and planning was first described by Mankovich et al [16] in 1990. Because of the 3D printing technology becoming more sophisticated and economically feasible, it gains popularity in clinical application. It has been reported in a Helsinki study that 114 models fabricated from 102 patients, 29% were performed due to malignant disease [17]. The benefits in providing preoperatively patient-specific osteotomy guides and reconstructive options are obvious [18]. So as this patient’s 3D skull model gave us a precise resection guide three dimensionally to avoid the violation of the vital anatomy such as orbital apex and skull base.
The invasion of the tumor into the nasopharynx that really complicated the treatment planning, yet we could lateralize the maxillectomy specimen to gain access to this region. Nevertheless, facial skeleton dissembling surgeries still with high morbidity, recently minimal invasive endoscopic approaches for nasopharyngectomy gained acceptable outcome [8-12,19,20]. In 2008 Ozer & Waltonen [21] first to described a preclinical study on a fresh-frozen cadaver to showed the feasibility of the transoral robotic approach to perform a complete nasopharyngectomy, including clival resection and internal carotid artery dissection through the division of soft palate for full nasopharynx exposure . Recent clinical study showed early results of robotic nasopharyngectomy with a high local control rate and the operating time was comparable to open surgery and low morbidities [22].
In conclusion, this report highlights the essence of surgical management of large para-nasal sinusoidal tumor that lend the surgeons to the extended medial maxillectomy; thus, familiarity with the details or modification of the procedure is invaluable.
- Lupinetti AD, Roberts DB, Williams MD, Kupferman ME, Rosenthal DI, et al. (2007) Sinonasal adenoid cystic carcinoma: the M.D. Anderson Cancer Center experience. Cancer 110: 2726-31. [Crossref]
- He S, Li P, Zhong Q, et al. (2017) Clinicopathologic and prognostic factors in adenoid cystic carcinoma of head and neck minor salivary glands: A clinical analysis of 130 cases. American J of Otolaryngology 38: 157-62.
- Rhee CS, Won TB, Lee CH, Min YG, et al. (2006) Adenoid cystic carcinoma of the sinonasal tract: Treatment results. Laryngoscope 116: 982-986. [Crossref]
- Thompson LDR, Penner C, Ho NJ, Foss RD, Miettinen M, et al. (2014) Sinonasal Tract and nasopharyngeal adenoid cystic carcinoma: A clinicopathologic and immunophenotypic study of 86 cases. Head and Neck Pathol 8: 88-109. [Crossref]
- Shah JP (2007) Surgical approaches to the oral cavity primary and neck. Int J Radiat Oncol Biol Phys 69: S15-18. [Crossref]
- Weisman R (1995) Lateral rhinotomy and medial maxillectomy. Otolaryngol Clin North Am 28: 1145-1156. [Crossref]
- Poetaker DM, Loehrl TA, Toohill RJ (2010) External medial maxillectomy. Operative Techniques in Otolaryngology 21: 107-110.
- Fisch U (1983) The infratemporal fossa approach for nasopharyngeal tumors. Laryngoscope 93: 36-44.
- Shu CH, Cheng H, Lirny JF, Chang FC, Chao Y, et al. (2000) Salvage surgery for recurrent nasopharyngeal carcinoma. Laryngoscope 110: 1483-1488. [Crossref]
- Nuss DW, Janecka IP, Sekhar LN, Sen CN (1991) Craniofacial disassembly in the management of skull-base tumors. Otolaryngol Clin North Am 24: 1465-1497. [Crossref]
- Morton RP, Liavaag PG, McLean M, Freeman JL (1996) Transcervico-mandibulo-palatal approach for surgical salvage of recurrent nasopharyngeal cancer. Head Neck 18: 352-358. [Crossref]
- Wei WI, Lam KH, Sham JS (1991) New approach to the nasopharynx: the maxillary swing approach. Head Neck 13: 200-207. [Crossref]
- Brown JS, Rogers SN, McNally DN, Boyle M (2000) A modified classification for the maxillectomy defect. Head Neck 22: 17-26. [Crossref]
- Martins C, Silva IEC, Campero A et al. (2011) Microsurgical Anatomy of the Orbit: The Rule of Seven. Anatomy Research International 1-14.
- Amit M, Binenbaum Y, Sharma K, Naomi R, Ilana R, et al. (2013) Adenoid cystic carcinoma of the nasal cavity and paranasal sinuses: A meta-analysis. J Neurological Surg 74: 118-125. [Crossref]
- Mankovich NJ, Robertson DR, Cheeseman AM (1990) Three-dimensional image display in medicine. J Digit Imaging 3: 69-80. [Crossref]
- Suomalainen A, Stoor P, Mesimki K, Kontio RK (2015) Rapid prototyping modelling in oral and maxillofacial surgery: A two-year retrospective study. J Clin Exp Dent 7: e605-612. [Crossref]
- Bosc R, Hersant, Carloni R, Niddam J, Bouhassira J, et al. (2017) Mandibular reconstruction after cancer: An in-house approach to manufacturing cutting guides. Int J Oral Maxillofac Surg 46: 24-31. [Crossref]
- Chen MK, Lai JC, Chang CC, Liu MT (2007) Minimally invasive endoscopic nasopharyngectomy in the treatment of recurrentT1-2a nasopharyngeal carcinoma. Laryngoscope 117: 894-896. [Crossref]
- Yoshizaki T, Wakisaka N, Murono S, Shimizu Y, Furukawa M (2005) Endoscopic nasopharyngectomy for patients wtith recurrent nasopharyngeal carcinoma at the primary site. Laryngoscope 115: 1517-1519. [Crossref]
- Ozer E, Waltonen J (2008) Transoral Robotic Nasopharyngectomy: A Novel Approach for Nasopharyngeal Lesions. Laryngoscope 118: 1613-1616. [Crossref]
- Tsang RK, To VS, Ho AC, Ho WK, Chan JY, et al. (2015) Early results of robotic assisted nasopharyngectomy for recurrent nasopharyngeal carcinoma. Head Neck 37: 788-793. [Crossref]









