Fabrication of fine copper wire in air by laser irradiation and micro-contact printing using stable copper complex as starting material has been investigated. A stable fine copper wire with denseness and smoothness was formed on glass substrate even in air by CO2 laser irradiation to a mixed complex film composed of glyoxylic copper complex and methylamine copper complex. The mixed-copper-complex solution was transferred to the substrate by micro-contact printing (μ-CP) using a poydimethylsiloxane (PDMS) mold, and fine copper wire with width of 5 μm was formed by laser irradiation. Resistivity of the copper film showed 1.95×10-5 Ω·cm at laser output of 5.6 W. This method enables the high-speed deposition of fine copper wiring in air by a printing process, indicating an inexpensive and useful process for fabricating copper wiring without high vacuum facility and heat-treatment under inert gas.
Glyoxylic acid copper complex, CO2 laser, Fine copper wire, Laser direct patterning, Micro-contact printing, Printable electronics
In recent years, “printable electronics,” that is, electronic devices produced by using a low-cost printing method, has attracted considerable attention [1-3]. Compared to the conventional method using high-vacuum facility such as sputtering method, vacuum evaporation method, and chemical vapor deposition (CVD), printable electronics is attracting attention as an innovative low-cost method for manufacturing electronic devices because it enables film formation by simple equipment under atmospheric pressure. Even in the case of forming metal wire, which is indispensable for electronic devices, the wire is formed by a printing method using fine metal particles. Although studies on the formation of gold and silver wires have been reported, the wires are formed by using a highly dispersed solution of gold or silver ultrafine particles by a method such as screen printing or inkjet printing [4-10]. However, gold and silver are precious metals, so using them is a problem from the viewpoint of cost reduction. Although silver has higher conductivity and is less expensive than gold, it suffers the problem of low migration resistance. For that reason, forming copper wire by using a dispersed liquid of particles of fine copper (which is a base metal with high conductivity) is being studied [11,12]. However, copper is extremely easily oxidized, so it is essential to perform treatment, such as synthesis of copper fine particles, preparation and handling of dispersion solutions of those particles, and heat treatment required for film curing, under an inert gas [13,14]. For that reason, the manufacturing process and equipment thereof are inevitably complicated, and that complication is one reason for increasing the manufacturing cost. Accordingly, the development of a stable and inexpensive copper-source compound and a simple process technology for forming copper wire using that compound is keenly awaited. Recently, formation of a copper film by using a stable solution composed of copper formate and 2-amino-2-methyl-1-propanol (AMP) as a copper source has been reported [15]; however, for conversion to copper, heat treatment at about 350°C under an inert gas is necessary. A method of forming a copper film with relatively good conductivity by irradiating a copper salt (as ink) with a high-intensity pulsed light has also been reported [16,17]. However, using such an irradiation method for forming a fine wire has not been investigated and reported.
Aiming to develop a simpler fabrication method for copper interconnects, we are studying the formation of high-speed fine interconnects in air by using stable copper-metal complexes and laser irradiation. We have reported two main findings from those studies so far: (i) laser irradiation onto a copper-complex film combining a glyoxylic acid bonded as a ligand causes stable metal copper to precipitate in air and (ii) it is possible to perform direct drawing (“direct patterning”) of copper wire by utilizing the phenomenon that the copper precipitates in the laser-irradiated part only [18]. With this method, it is possible to form fine copper wire at high speed by laser irradiation of a copper metal complex that is stable and easy to handle in air. As a result of that advantageous feature, the method is attracting attention as a simple and inexpensive means of forming copper wire that neither requires high-vacuum facility nor treatment under an inert gas. However, the line width of the fabricated copper wire depends on the irradiation aperture of the laser, which is limited to about 200 μm.
In the present study, aiming at forming finer wire, we focused on a method of forming fine copper wire in air by using laser irradiation and micro contact printing (μ-CP). Although a glyoxylic-acid copper complex is used as a starting material, it was found that when an amine-series copper complex is added to the former complex as the second component, a more uniform and finer copper wire can be formed. Using this mixed solution of copper complexes as a copper source, we succeeded in forming fine copper wire (with width of 5 μm) in air by using μ-CP and laser irradiation.
Synthesis of glyoxylate-copper (GACu) complex
Glyoxylic acid (4.5 mmol, Tokyo Chemical Inc.) was dissolved in 5 ml of distilled water (H2O) and adjusted to pH 7 by adding 10 wt% of sodium hydroxide (NaOH, Kanto Chemical Co.) aqueous solution. Then, CuSO4・5H2O (4.5 mmol, Kanto Chemical Co.) dissolved in 5 ml of H2O was added to that solution. After the mixture was stirred for three hours, the (blue) precipitate was filtered and washed with sufficient H2O. The blue precipitate was then dried under reduced pressure (yield (weight): 0.50 g; yield (proportion): 65.7%).
Synthesis of methylamine-copper (MACu) complex
To prepare a methylamine-copper (MACu)-complex solution (1.0 M), 6.0 mL of 40% methylamine-methanol solution (WAKO Chemical Co.) was added to 1.3 g of copper-formate tetrahydrate (WAKO Chemical Co.) and stirred (with warming) for one hour. The complex solution was gradually concentrated, the solvent was removed, and blue MACu precipitates were obtained by drying.
Preparation of copper-complex mixed solution
To prepare a GACu solution (1.0 M), 4.0 mL of ethanol and 2.0 mL of 2-aminoethanol were sequentially added to 1.0 g of GACu and stirred for one hour. In addition, to prepare a MACu-complex solution (1.0 M), 6.0 mL of a 40% methylamine-methanol solution was added to 1.3 g of copper-formate tetrahydrate and stirred for one hour. To prepare a mixed solution (1.0 M) of GACu and MACu complexes, the MACu complex was added to the GACu solution and stirred for 30 minutes.
Thin-film preparation and laser irradiation
The GACu/MACu mixed solution was spin coated (rotational speed: 2000 rpm; 30 s) onto an alkali-free glass substrate (20 × 25 × 1.5 mm)) and then dried at 80°C for 10 min. The output of a carbon-dioxide (CO2) gas laser (SUNX LP-300; wavelength: 10.6 μm) was changed as the thin film was irradiated. During the irradiation, the laser focus was swept at a sweep rate of 20 mm/s. After laser irradiation, non-irradiated portions were removed from the thin film by etching with a mixed solvent of ethanol/H2O (at ratio of 1: 1) or pure water. The film was then washed with acetone and dried at room temperature.
Formation of copper micro-wire using μ-CP
Polydimethylsiloxane (PDMS; Dow Corning Toray Co.; SILPOT 184) was used as a μ-CP stamper (mold). A PDMS solution was poured onto a fine pattern formed on a silicon wafer and thermally cured. Releasing the cured PDMS revealed the PDMS with the fine pattern transferred to it. The finely patterned PDMS was used as a stamper for μ-CP. A film of copper-complex mixed solution (1.7 M) was formed on a glass slide by spin coating (3000 rpm for 30 s). Then, the μCP stamper (mold), on which the fine pattern was formed, was adhered to the complex film, and a complex solution was deposited on the pattern. The complex solution deposited on the PDMS pattern was then transferred to another glass slide and prebaked at 80°C for 10 minutes to form a fine pattern composed of copper-complex solution. By irradiating the copper-complex fine pattern with a CO2 laser (at sweep speed of 20 mm/s), the copper complex was converted to copper in the form of fine copper wire.
Evaluation
Absorption by the synthetic complex was measured by visible-ultraviolet absorption spectrometer (Shimadzu UV-2540). The thermal-decomposition characteristics of the synthetic complex were measured by thermogravimetric differential thermal analysis (TG-DTA; Shimadzu TGA-50) and thermogravimetric mass spectrometry (TG-MS; RIGAKU TG 8120 and Shimadzu GCMS-QP 2010). The crystal structure of the thin film was measured by thin-film X-ray diffractometer (RIGAKU RINT-TTR III, Smart Lab). The surface condition of the thin film was observed by SEM (KEYENCE VE-8800 and JEOL SM-7610 F). The resistivity of the thin film was measured by the four-needle method (Mitsubishi MCP-T360).
Structure and properties of GACu and MACu
The molecular structures of GACu and MACu are shown schematically in (Figure 1). As for the molecular structure of GACu, one GA and two H2O molecules coordinate to a copper atom, and as for the molecular structure of MACu, four methylamines coordinate to a copper atom. Both copper complexes have high solubility in solvents such as amino alcohols.
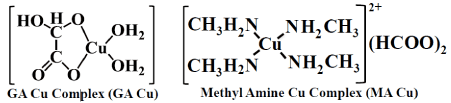
Figure1. Molecular structures of glyoxylic acid copper complex (GACu) and methylamine copper complex (MACu).
The absorption spectrum in the visible-light region when GACu is dissolved in amino alcohol is shown in Figure 2 (c). For comparison, the absorption spectra of other copper complexes in which each of ethylene diamine (a), amino alcohol (b), and oxalate (c) is coordinated with copper are also shown. If the coordination atom coordinated with copper is focused on, it is clear that GACu becomes CuNO3 (Figure2 (c)). The ethylene-diamine complex shown as a comparison target is CuN4, the amino-alcohol complex is CuN2O2, and the oxalate complex is CuO4. The position of the absorption peak in the absorption spectrum of each complex shifts to the long-wavelength side in the ascending order of wavelength as CuN4 (598 nm) > CuN2O2 (625 nm) > CuNO3 (647 nm) > CuO4 (709 nm). Since the spectrochemical series of coordination atoms is represented as N>O [19], the spectrum systematically changes to the longer-wavelength side as nitrogen is replaced with oxygen. Since the absorption peak of GACu (CuNO3) is intermediate between CuN2O2 and CuO4, GACu is presumed to be a structure in which the two H2O in the molecular structure of GACu are substituted and coordinated by the amino alcohol. On the other hand, MACu has an absorption peak near 600 nm, which is almost the same as that of the ethylene-diamine complex. This fact suggests that MACu has a CuN4 structure; in other words, MACu has a structure in which methylamine is bonded to a copper atom at four nitrogen-atom positions.
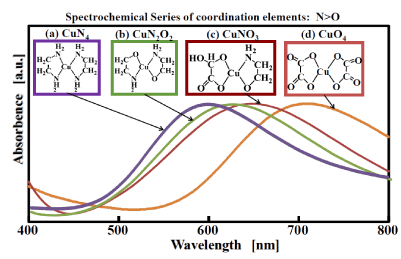
Figure 2. Absorption spectra of GACu complex in ethanolamine and other Cu complexes.
The results of the thermal-analysis characterization (TG-DTA and TG-MS) of the GACu and MACu complexes are shown in (Figures 3 and 4), respectively. In Figure 3(a), the weight-loss ratio of GACu as a whole was 65.1% (at 280°C), which roughly agrees with the theoretical value (66.5%) (namely, when the ligand was combusted and removed). Weight loss mainly occurs in two stages: one at 100 to 140°C and another at 200 to 280°C. In the first weight-loss step (at 100 to 140°C), molecules with molecular weight of 18 are generated in large amounts (Figure3(b)). The TG result in (Figure 3) shows that the weight-reduction ratio is 10.3% in the region between 100°C and 140°C. This ratio is close to the reduction ratio of one molecule of water (9.5%) in GACu. Further, an endothermic peak is observed in the TG-DTA curve. It is therefore considered that evaporation of one molecule of water occurs in the first step. At the second weight-loss step, molecules with many molecular weights, namely, 18, 28, and 44, are generated (Figure3(b)). These molecular weights are consistent with water, carbon monoxide, and carbon dioxide, respectively. In the TG result in (Figure 3), weight reduction ratio between 140°C and 200°C is 54.8%. This value is consistent with the reduction ratio of one molecule of water and the glyoxylic-acid component (54.8%). Furthermore, an exothermic peak is observed in the DTA result, so it is considered that organic matter (glyoxylic acid) is being combusted.
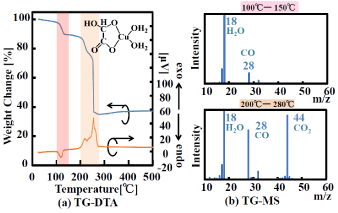
Figure 3. TG-DTA curves and mass spectra (TG-MS) of GA Cu.
On the other hand, in the case of MACu, as shown in Figure 4(a), a large weight loss is observed from 160°C to 200°C, and the weight loss is completed around 240°C. The total weight loss is 86.20%, which is slightly higher than the theoretical reduction ratio of 77.13%. Although the reason for this result is not clear, since MACu precipitate powder is obtained by drying the residue formed by evaporating a reaction solvent, it is possible that it contains a small amount of solvent, methylamine, etc. An endothermic peak is observed from 160°C to 200° C, and an exothermic peak at is observed from 200°C to 230°C (Figure 4(a)). From the TG-MS results (Figure4(b)), a CH3NH2N (MW: 31.06) peak is observed at m/z = 31 from 160°C to 200°C, and peaks are observed at m/z = 44, 28, and 18, namely, CO2, N2, and H2O, respectively. Since methylamine coordinated to copper has a low boiling point (b.p.: -6°C), when the bond breaks, an endothermic peak (due to vaporization to a gaseous state) appears and it burns immediately. Since an exothermic peak is observed between 200 and 240°C, it is supposed that burning of CH3NH2 and formate ions (which is a counter anion) occurs in that temperature range. In the TG-MS spectrum in this temperature range, peaks at m/z = 44, 28, and 18 are observed, which are considered to be CO2, N2, and H2O produced by burning of the ligand. Elimination of the ligand in the case of MACu occurs at a lower temperature than that in the case of GACu, suggesting that copper precipitation is possible by applying lower energy.
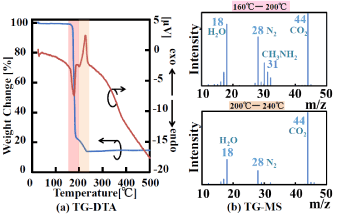
Figure 4. TG-DTA curves and mass spectra (TG-MS) of MA Cu.
Formation of copper thin film in air by laser irradiation to mixed-copper-complex film
As for GACu, the decomposition process by which copper precipitates by laser irradiation is given by the following equation. When GA (with a strong reducing property) desorbs from Cu2+, Cu2+ is reduced to copper. Since GA becomes a gas (such as CO2, CO, and H2O) and is removed, copper of high purity precipitates. Copper can thus be expected to precipitate at lower energy by adding MACu (having a lower decomposition temperature) to GACu.
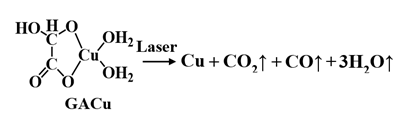
The process for forming the copper thin film by irradiating the mixed-copper-complex film with a laser is shown schematically in (Figure 5). A solution composed of an amino-alcohol solution of GACu and a methanol solution of MACu mixed at an arbitrary ratio was prepared, and the solution was applied to a glass substrate and then laser irradiated in air. The copper wires precipitated by laser irradiation remain on the substrate by etching with water or ethanol, which dissolves Cu complexes (GACu and MACu).
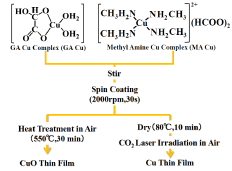
Figure 5. Cu film formation process using mixed solution of GA Cu and MA Cu.
Surface morphologies of copper precipitations (fine wire) obtained using the mixed complex solution of GACu: MACu (laser irradiation condition: laser power, 2.4 W; sweep speed, 20 mm/s) obtained for several composition ratios of GaCu:MACu (10:0, 8:2, 6:4, 5:5, 4:6, 2:8, and 0:10) are shown in (Figure 6). Although copper precipitation was observed for all composition ratios, when the composition ratio was 6:4 or 5:5, a copper wire with a flat and uniform surface was obtained. For the composition ratios at which the proportion of GACu is high (i.e., 10:0 and 8:2) and those at which the proportion of MACu is high (i.e., 4:6, 2:8, and 0:10), laser-sweep traces on the surface of the copper film can be observed, and the surface flatness and uniformity tend to worsen. These observations indicate that there is an optimal mixture ratio of GACu and MACu.
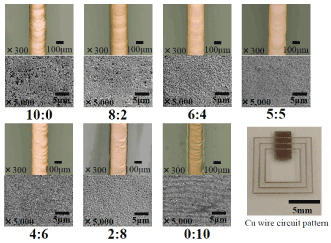
Figure 6. Surface morphology of Cu fine wire using mixed complex solution of GACu: MACu (Laser irradiation condition: laser power 2.4W, sweep speed 20mm/s).
The X-ray diffraction spectrum of the thin film with a composition ratio of 5:5 is shown in (Figure 7). For comparison, the spectra of GACu and the heat treated film in air at 550°C are also shown. The XRD spectrum of the copper-complex film heat treated in air shows peaks at 2θ = 35.5° (1, 1, -1), 38.7° (1, 1, 1), and 48.8° (2, 0, -2). It is confirmed from the spectra that these peaks represent copper (II) oxide (JCPDS No. 9). On the other hand, when the copper-complex film was subjected to atmospheric laser treatment, peaks appear at 2θ = 43.3° (1, 1, 1), 50.4° (2, 0, 0), and 74.2° (2, 2, 0). These peaks coincide with the peaks for cubic-crystal metallic copper (JCPDS No. 225), revealing that copper crystals were precipitated by the laser irradiation in air.
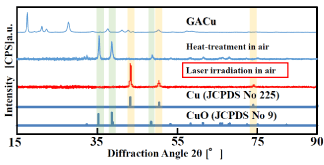
Figure 7. X-ray diffraction patterns of laser-irradiation film in air, heat-treated film at 550°C in air and GA Cu complex.
Influence of laser output on surface condition and resistance of copper film
Changes in laser power have a significant influence on the conductivity and surface condition of a copper film. Resistance and change in surface condition when the output of the laser irradiation onto the complex film with GACu:MACu ratio of 5:5 are shown in Figure 8. According to the figure, at output intensity of 2 W or less, the conversion to copper was insufficient, and the film was a mixture of metal complex film and copper. As a result, this resistance indicates the film is insulating. The film becomes conductive at 2.4 W and above, and resistivity greatly decreases at 3.6 W and above. In other words, as output increased, resistance decreased, reaching 1.95×10-5 Ω·cm at output of 5.6 W. Above 5.6 W, resistivity does not significantly change. The SEM images of the surface state of the films obtained when laser-irradiation output was changed from 2.4 W, to 3.6 W, and to 5.6 W are shown in Figure 8 (a), (b) and (c), respectively. Many pores were generated in the film formed at 2.4 W. The abundance of the pores decreased with increasing laser-irradiation power, and in the case of 5.6 W, a state in which the copper particles were melted and connected was observed. It is presumed that as laser-irradiation power increases, the continuity of the copper particles increases, and the resistance decreases.
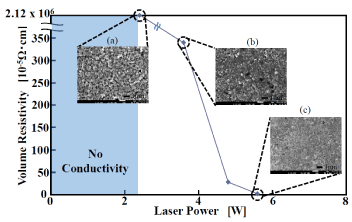
Figure 8. Relationship between laser power and resistivity and surface SEM images.
As for laser irradiation of the GACu-only (10: 0) film, the film was conductive at 4 W or more, and resistivity was 3.0×10-5 Ω·cm at 12 W [18]. By laser irradiation of the mixed complex film composed of GACu and MACu, a film exhibiting the same degree of conductivity formed with about half of that laser output was obtained. Addition of MACu to GACu makes it possible to obtain a dense and uniform copper film while using less irradiation energy.
Time-dependent changes of surface resistance of the GACu:MACu=5:5 copper film fabricated at each output laser power are shown in (Figure 9). The surface resistances of the films immediately after preparation, after 10, 50, and 110 days are shown. The surface resistance of the copper film fabricated with lase power of 6 W does not change even after the passage of 110 days; in other words, its resistance is stable. In contrast, the resistance of the film fabricated with lase power of 2.4 W increases with time. For 3.6 W or higher, the resistance change is minute in all cases. The surface observations by SEM also confirm the uniformity and denseness of the surface of the copper film precipitated as the laser output is increased, and it was found that the film formed with laser power of 6 W is stable.
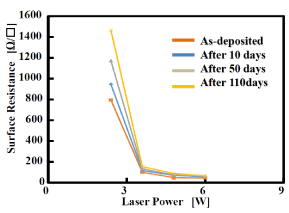
Figure 9. Relationship between surface resistance and laser power and changes of surface resistance in time lapse.
Formation of fine copper wire using μ-CP and laser irradiation
The process for forming copper micro-wire by μ-CP and laser irradiation is outlined in Figure 10. The PDMS (polydimethylsiloxane) mold used for μ-CP was fabricated using a silicon substrate (on which a fine pattern was formed) as a mold material. In the PDMS, smooth lines with width of 5 and 10 μm are formed uniformly. For the mixed copper-complex solution, a solution with a concentration of 1.7 M was used to increase adhesion to PDMS. PDMS is adhered to the mixed complex film formed on the glass substrate, and a complex film is deposited on the PDMS. Then, the PDMS mold is brought into contact with the substrate to transfer the complex film. After the transferred complex film is dried, laser irradiation is performed to precipitate copper.
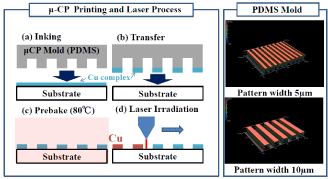
Figure 10. Cu fine wire formation process by μ-CP and laser irradiation.
The change in the surface condition of the μ-CP film (10μm pattern) after laser irradiation is shown in (Figure 11). At laser power of 1.8 or 2.0 W, particulate copper was precipitated, and the copper particles migrated to the grooves of the pattern, so the fine pattern disappeared. At 2.2 or 2.4 W, a copper pattern with a width of 10 μm is formed. As for the surface condition of those patterns, it is observed that the copper particles are coupled by melting. It is conceivable that the minute copper particles precipitated by laser irradiation melt instantaneously at higher than a defined laser output (2.2W or higher) and changes to more stable and larger copper particles. It is presumed that when the laser output is low, copper particles precipitate; however, no energy is available to instantaneously promote melting and coupling between the copper particles, so their adhesion to the substrate is weak, and the copper particles spread between the lines of the pattern. A stable copper pattern was observed to form at 2.4 W or higher.
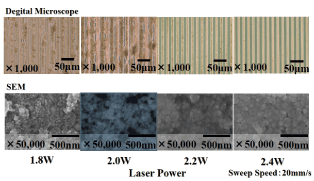
Figure 11. Surface of copper fine wires using μ-CP (width 10μm) and laser irradiation.
The surface condition and cross-sectional structure of a 5-μm-wide copper pattern is shown in (Figure 12). It is understood from the images that a copper line-and-space pattern with width of 5 μm is accurately formed. It is also understood that copper particles with size of about 50 nm are brought into contact with the substrate surface and melted, so that a larger copper particles are formed. Moreover, the thickness of the copper film is about 94 nm, and the copper film is uniformly formed on the glass substrate. In regard to the copper-patterned films with width of 5 and 10 μm, a tape-peeling test (JIS K5600-5-6) did not show film peeling, indicating that the adhesive force between the copper film and the substrate was high.
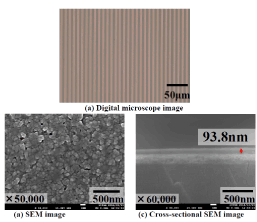
Figure12. SEM and digital microscope images of Cu fine wire with 5μm width.
Mechanism of copper deposition by laser irradiation
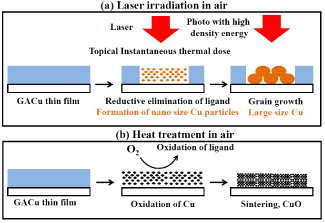
The mechanism by which a copper thin film is formed by laser irradiation in air is shown schematically in (Figure 13). A CO2 laser (with a wavelength of 10.6 μm), namely, an infrared laser (a kind of thermal laser), can be used to apply heat (with a high energy density) locally and instantaneously to the irradiated part. That heat instantaneously detaches and eliminates the ligand. At that time, electrons are donated to Cu2+ ions by reductive elimination of the ligand and reduced to copper atoms. It is assumed that since the copper immediately after the reductive formation is formed as minute particles, the melting point of the former copper is lower considerably compared with that of the bulk copper. The size effect reducing melting point is well-known to nano-particle [20,21]. It is considered that the fine copper particles are melted by the heat provided by the laser irradiation, and they grow faster than possible by being oxidized into more stable large copper particles. Mixing MACu with GACu when generating copper produces thermal decomposition with lower energy than that with GACu since MACu decomposes at lower temperature than that of GACu. Consequently, copper crystals produced from GACu are easily grown since fine copper particles (formed from MACu) operate as a crystal nucleus. This fact is presumed to be the reason that the GACu/MACu mixed-complex film is formed by lower laser output than that for the GACu single film.
2021 Copyright OAT. All rights reserv
Figure 13. Mechanism of Cu thin film formation by laser irradiation, (a) laser irradiation in air, (b) heat treatment in air.
On the other hand, in the case of heat treatment, thermal energy is gradually applied to the entire film by convection. It is therefore presumed that the fine copper particles generated when the ligand is reductively eliminated immediately react with oxygen in the air diffused into the film without grain growth, thereby forming copper oxide.
It was found that a stable fine copper wire can be formed even in air by CO2 laser irradiation to a mixed complex film composed of GACu and MACu. As a result of mixing MACu with GACu, a dense and uniform copper film is precipitated with about half the laser power than that required for forming a copper film from a GACu single film. The mixed-copper-complex solution was transferred to the substrate by μ-CP using a PDMS mold, and fine copper wire with width of 5 μm could be formed by laser irradiation.
By means of this method, it is possible to form a fine copper wire in air by using a stable copper complex as a raw material. In addition, a special high-vacuum facility is not required, and fine copper wire can be formed at high speed by laser processing. This advantageous feature will make it possible to reduce the cost of the process technology used for forming copper wire.
- Perelaer JS, Mager PJ, Soltman D, Volkman D, Subramarian SK, et al. (2010) Printed Electronics, J. Mater. Chem 20: 8446.
- Yokoyama M, Kamata T (2008) Advanced Science and Technology for Printable Organic Electronics, CMC Publishers.
- Minemawari H, Yamada T, Matsui H, Tsutsumi J, Haas S, et al. (2011) Inkjet printing of single-crystal films Nature 475: 364.
- Fukuda K, Sekina T, Kobayashi Y, Takeda Y, Shimizu M, et al. (2012) Organic integrated circuits using room-temperature sintered silver nanoparticles as printed electrodes Organic Electronics 13: 3296.
- Tokuno T, Nogi M, Karakawa M, Jiu JTT, Nge Y, et al. (2011) Fabrication of silver nanowire transparent electrodes at room temperature Nano Res 4: 1215.
- Hosel M, Krebs FC (2012) Large-scale roll-to-roll photonic sintering of flexo printed silver nanoparticle electrodes J. Mater. Chem 22: 15683.
- Nakamoto M, Kashiwagi Y, Yamamoto M (2005) Synthesis and size regulation of gold nanoparticles by controlled thermolysis of ammonium gold(I) thiolates in the absence or presence of amines Inorg. Chim. Acta 358: 4229.
- Suganuma K, Sakamoto S, Kagami N, Wakuda D, Kim KS, et al. (2012) Low-temperature low-pressure die attach with hybrid silver particle paste Microelectron Relib 52: 375.
- Morita T, Ide E, Yasuda Y, Hirose A, Kobayashi KF (2008) Study of bonding technology using silver nanoprticles Jpn. J. Appl. Phys 47: 6615.
- Perelaer J, Abbel R, Wtinscher S, Jani R, van Lammeren T, et al. (2012) Roll-to-roll compatible sintering of inkjet printed features by photonic and microwave exposure: From Non-Conductive Ink to 40% Bulk Silver Conductivity in Less Than 15 Seconds Adv. Mater 24: 2620.
- Kim Y, Yoo BW, Anthony JE, Park SK, (2012) Controlled deposition of a high-performance small-molecule organic single-crystal transistor array by direct inkjet printing Adv. Mater 24: 497.
- Choi Y, Lee JH, Kim SJ, Yoon DH, Byun YH (2012) Highly conductive polymer-decorated Cu electrode film printed on glass substrates with novel precursor-based ink and paste J. Mater. Chem 22: 3624.
- Ryu JG, Kim HS, Hahn HT (2011) Reactive sintering of copper nanoparticles using intense pulsed light for printed electronics J. Electron. Mater 40: 42.
- Ishizaki T, Watanabe R (2012) A new one-pot method for the synthesis of Cu nanoparticles for low temperature bonding J. Mater. Chem 22: 25198.
- Shin D-H, Woo S, Yem H, Cha M, Cho S, et al. (2014) A self-reducible and alcohol-soluble copper-based metal-organic decomposition ink for printed electronics Appl. Mater. & Interfaces 6: 3312.
- Wang BY, Yoo TH, Song YW, Lim DS, Oh YJ, (2013) Cu ion ink for a flexible substrate and highly conductive patterning by intensive pulsed light sintering Appl. Mater. & Interfaces 5: 4113.
- Araki T, Sigahara T, Jiu J, Nagao S, Nogi M, et al. (2013) Cu salt ink formulation for printed electronics using photonic sintering Langmuir 29: 11192.
- Ohishi T, Kimura R (2015) Fabrication of copper wire using glyoxylic acid copper complex and laser irradiation in air Materials Sciences and Applications 6: 799.
- Shriver and Atkins Inorganic Chemistry (2006) Fourth Edition, Oxford University Press.
- Buffat PH, Borrel JP (1976) Size effect on the melting temperature of gold particles Phys. Rev. A 13: 2287.
- Kim HS, Dhade SR, Shim DE, Hahn HT (2009) Intense pulsed light sintering of copper nanoink for printed electronics Applied Physics A 97: 791.

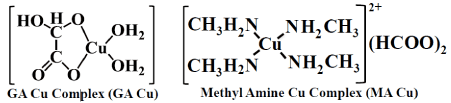












 2021 Copyright OAT. All rights reserv
2021 Copyright OAT. All rights reserv