Purpose: Poorly water-soluble drugs when given orally experience limited dissolution due to their low solubility in aqueous solutions. This study aimed to investigate the dissolution enhancement using self-emulsifying drug delivery systems (SEDDS) for Indapamide, a Biopharmaceutical Classification System Type 2 drug. Stability of SEDDS was also assessed.
Methods: Drug containing lipid-based systems that could form SEDDS were prepared with vehicles including Labrafil®M1944CS and Labrasol with or without Capryol™90 or Solutol®HS15. The key quality attributes of SEDDS were determined in terms of size distribution and drug dissolution profiles. Other properties evaluated were critical micelle concentration (CMC), zeta potential and solubility profiles.
Results: SEDDS formed negatively charged dispersions of typically 170-250nm and their CMC were <110mg/L. All SEDDS achieved rapid and significantly higher drug release, which were maintained over 90min when compared to unprocessed drug powder. Dissolution enhancement and size distribution profiles of SEDDS stored at 25 and 40ºC were preserved. Enhancement effect of SEDDS was contributed to formation of stable nano-sized dispersions, high drug solubility in SEDDS and low CMC that withstood dilution by dissolution medium.
Conclusions: Lipid-based systems with Labrasol, Labrafil®M1944CS and Capryol™90 produced SEDDS that led to a significant dissolution enhancement of Indapamide regardless of storage conditions.
self-emulsifying, hydrophobic drug, indapamide, dissolution, size distribution.
Drug particle that presents with limited aqueous solubility tend to exhibit poor dissolution profile. This can hamper oral drug absorption that has been related to issues of low and unpredictable bioavailability and lacking dose proportionality [1]. Hence dissolution enhancement for these drugs is crucial to avoid therapeutic failures. Key enhancement approaches include solubility improvement such as via salt formation and dissolution rate enhancement such as forming solid dispersions or self-emulsifying drug delivery system (SEDDS), each approach has its cons and pros despite showing enhancement for certain hydrophobic drugs [2-4]. Indapamide is a thiazide-like diuretic with antihypertensive effects. It is a weak acid and the partition coefficient for indapamide is higher than other diuretic agents including furosemide, hydrochlorothiazide and chlorthalidone [5]. It falls under Biopharmaceutical Classification System (BCS) Type 2 (low solubility and high permeability) [6]. Producing amorphous forms of Indapamide by spray drying [7,8], quench-cooling of the melt, cryogenic grinding and room temperature milling [9,10] have been shown to improve drug solubility against its crystalline form. And drug precipitation could be avoided by adding small amount of hydroxypropyl methylcellulose. Lipid-based systems that generate SEDDS have shown success with several marketed products being adopted [11]. This study aimed to investigate the feasibility of SEDDS as solubility and dissolution enhancing approach for Indapamide. In order to produce lipid-based systems, Labrasol with Hydrophilic-Lipophilic Balance (HLB) of 12 and Labrafil®M1944CS (HLB 9) have been chosen as the key components while CapryolTM90 (HLB 5) or Solutol®HS15 (HLB 14-16) as the third component. These vehicles and their mixtures have previously been reported to enhance drug dissolution profiles [12]. Effect of blending these vehicles in different drug to lipid ratios to produce lipid-based systems, which were suitable as SEDDS for Indapamide, were examined. Performances of SEDDS were analysed based on their size, charge, morphology, solubility and dissolution profiles. Furthermore, the critical micelle concentration (CMC) of SEDDS was determined. The stability profiles of SEDDS were also examined in terms of size distribution and dissolution profiles.
Indapamide, phosphotungstic acid (PTA), osmium tetraoxide (OST), acetonitrile, ethanol and potassium dihydrogen orthophosphate were obtained from Sigma Aldrich (Dorset, UK). Labrasol, Labrafil®M1944CS and Capryol™90 were purchased from Gattefosse (Binfield, UK). Solutol®HS15 was obtained from BASF (Cheshire, UK). All chemicals were of analytical grade and used as obtained.
Lipid-based vehicle systems were prepared by blending Labrafil®M1944CS and Labrasol with and without CaproylTM90 or Solutol®HS15. Indapamide was dissolved in the lipids at a specific drug to lipid ratio at 60°C (Table 1). The mixture was cooled to 25°C and then vortexed until clear liquids were obtained. For sample characterisation, dissolution study using USP dissolution apparatus II (Caleva Ltd., Dorset, UK) was performed on unprocessed Indapamide powder, marketed generic tablets and SEDDS (all samples contained 5mg of drug). Each sample was placed in 900mL water (as dissolution medium) at 37±1°C and stirred at 100rpm. A 10mL sample was withdrawn at fixed intervals for up to 90min and an equal amount of distilled water was replaced. Samples were analysed for drug levels by a high-performance liquid chromatography (HPLC) assay (Figure S1). Solubility study was conducted by placing an excessive amount of drug powder in either individual excipients or lipid-based systems. Samples were mixed and equilibrated for 48h at 37oC. After centrifugation, appropriate amount of supernatants were diluted in up to 50 ethanol before HPLC analysis. Lipid-based systems (same quantity used as dissolution test) were diluted in water forming SEDDS, then size distribution and zeta potential were determined using Malvern Zetasizer Nano ZSP (Cambridge, UK) [13] while the morphology of SEDDS was examined by transmission electron microscopy (TEM) (Hitachi H700, Japan) after negative staining by 2% PTA and positive staining by 2% OST [14]. Surface tension values of diluted SEDDS were determined by a tensiometer (Attension Sigma 700/701, Sweden) attached to a platinum ring from 1g/L stocks [15]. Following this, the critical micelle concentration (CMC) values of SEDDS were determined. The dissolution and size distribution profiles were also determined after three- and six-months of stability tests for lipid-based systems stored at 40°C and 25°C, respectively. All experiments were repeated trice. One-way ANOVA was conducted to examine whether there is statistically significant difference among the groups and Tukey HSD was selected as Post Hoc test. The significance level used was 0.05.
Table 1. Formulation composition of lipid-based systems. *substituted with Solutol®HS15
Sample ID |
Labrafil® M1944CS (%) |
Labrasol (%) |
Capryol™90 (%) |
Drug/ lipid ratio |
C110 |
25 |
65 |
10 |
1/10 |
C120 |
25 |
65 |
10 |
1/20 |
C130 |
25 |
65 |
10 |
1/30 |
S130* |
25 |
65 |
10 |
1/30 |
B130 |
30 |
70 |
0 |
1/30 |
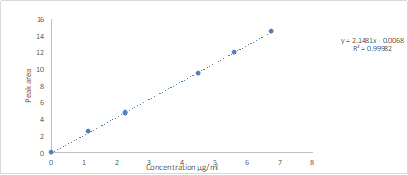
Figure S1. Indapamide calibration curve (Values were presented as mean and SD)
Determination of drug concentration by HPLC: The drug levels of were analysed after a separation process by High-performance liquid chromatography (HPLC), using Agilent 1290 Infinity series LC system with a binary pump (G4220A). The HPLC system was attached to a reversed phase column (4.6 x 150mm, Hichrom 5µm, C8 type, Hichrom, UK). The mobile phase was made of acetonitrile/10mM phosphate buffer pH 3 at 38/62 (v/v). Flow rate of the mobile phase was set to 1mL/min. The injection volume was 1µl and the run time was 7min for each sample. An UV-Vis detector (VWD G1314E) that was attached to HPLC was used to detect Indapamide at 242nm. Drug standards ranging between 1.12 and 6.72mg/mL were used. Following integration of drug peak areas, calibration plot and its equation was obtained (Fig. S1). The limit of detection was 0.14mg/mL and limit of quantitation was 0.54mg/mL.
Indapamide solubility in individual excipients was found to be the highest in Labrasol and the lowest in Labrafil®M1944CS (Figure 1), and the findings were consistent to earlier study [12]. The highest drug solubility was achieved with lipid-based system consisting of 10% CapyrolTM90. On the contrary, by substitution of 10% CapyrolTM90 for 10% Solutol®HS15 with the highest HLB, drug solubility was lower due to a slight increase in overall hydrophilicity of the system. These lipid-based systems achieved HLB values ~11 and they were considered superior to individual vehicles except for Labrasol. They improved Indapamide solubility and the solubility enhancement was well exceeding the target drug content, which was 5mg. Moreover, solubility enhancement is considered significant comparing to drug solubility in water alone, where the reported drug solubility in water ranged 81-109mg/L [10]. Hence, it is anticipated that drug partition would occur mainly into these lipid-based systems rather than water.
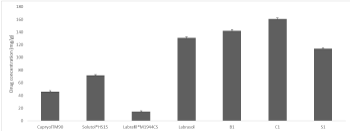
Figure 1. Solubility profiles of Indapamide in lipid-based systems and individual excipients. Data presented as mean and SD (n=3). Excipients composition of SEDDS can be found in Table 1: B1 (as in B130); C1 (as in C110, C120 or C130); S1 (as in S130)
The lipid-based systems were then prepared by using different drug to lipid ratios (1/10, 1/20, 1/30) initially and stored at 25°C. Periodically, the samples were examined visually. Samples were excluded if they phase separated. Those remaining as clear or transparent liquids were considered as stable and were selected for further analysis (Table 1). The mean sizes of SEDDS were in the range of 170-250nm (Figure 2), indicating the formation of dispersed nanostructures. This is due to relatively high HLB values of these lipid-based systems and low free energy requirement for self-emulsification to occur [16]. SEDDS with 10% Solutol®HS15 (batch ID=S130), which is considered most hydrophilic, exhibited the smallest size (p<0.05) while SEDDS devoid of the third component (ID=B130) possessed the highest polydispersity index (PDI) with a less uniform size distribution. Hence, incorporating Solutol®HS15 or CapryolTM90 as co-surfactant that changed interfacial fluidity was found to be beneficial to self-emulsification process [16].
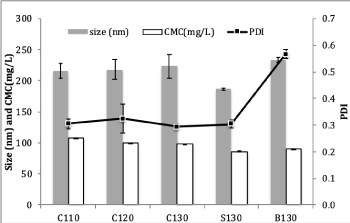
Figure 2. Size, PDI and CMC of SEDDS. Data presented as mean and SD (n=3). Mean sizes were represented by grey bars, mean CMC values by white bars and line represented PDI
Aggregation of surface-active agents occurs when their bulk concentrations reach a critical point, which is referred to as CMC. The dispersed nanostructures of SEDDS following dilution of lipid-based systems are advocated to exhibit similar properties, where these droplets are stabilised by a thin layer of surfactants surrounding the oil cores (consisting dissolved drug molecules) that lowers the interfacial energy and induces either charge or steric effect [12]. Negative zeta potential values ranging from -29 to -36mV were observed for all SEDDS, where SEDDS with 10% CapryolTM90 (ID=C110) at drug to lipid ratio of 1/10 gave the most negative value and the values declined as drug to lipid ratios increased (Table S1). As Indapamide behaves as a weak monoprotic acid, it can contribute to small increment in zeta potential values as the amount of dissolved drug increased in SEDDS (5). Images from TEM showed that nanostructures in SEDDS were round to oval in shape after negatively stained with PTA, where the droplet appeared bright in contrast to the surrounding shell, which was darker. This observation was further confirmed by applying a positive stain using OST where oil cores of the droplets were very dark imparting higher contrast level against the shells (Figure 3) [14]. CMC values of SEDDS were in the range of 86-108mg/L (Figure 2), consistent with ranges previously reported [4]. The highest CMC was seen in SEDDS with the smallest drug to lipid ratio (1/10; ID=C110), which presented with the largest quantity of dissolved Indapamide. The lowest CMC was observed in SEDDS with 10% Solutol®HS15 (ID=S130) where droplet size of SEDDS was the smallest. When comparing the effect of lipid compositions, it was found that there was no significant difference of CMC values among SEDDS at the same drug to lipid ratio. Low CMC is associated to greater tendency to produce micelles for a given system. This may lead to a lesser risk of failure, as the system will be less prone to dilution effect that can result in drug precipitation [2,4].

Figure 3. Typical images of SEDDS from transmission electron microscopy. (a) S130 negatively stained at 40000x; (b) S130 positively stained at 30000x
Table S1. Zeta potential of SEDDS. Values were represented as mean and Standard deviation
ID |
Zeta potential (mV) |
Standard deviation |
C110 |
-36.6 |
1.86 |
C120 |
-30.2 |
0.61 |
C130 |
-28.9 |
1.27 |
S130 |
-33.2 |
0.9 |
B130 |
-32.5 |
2.08 |
Drug dissolution profiles of SEDDS alongside unprocessed powder and marketed tablets were presented (Figure 4). As expected, all SEDDS attained rapid and high drug release after exposure to the dissolution medium as opposed to unprocessed powder and marketed tablets where drug dissoluion occurred more gradually [17]. The overall drug release for unprocessed drug, marketed tablet and SEDDS were 47%, 91% and 97-98%, respectively, where dissolution enhancement from SEDDS was significantly higher (p<0.05). Percentages drug release of SEDDS were maintianed over the study period, confirming that SEDDS not only accomplished its solubilisation effect but also showed a ‘supersaturation effect’ as high level of drug re-precipitation was not observed in SEDDS [18]. This was not only contributed to blending Labrasol with other surfactants [19,20] but also high drug solubility with low CMC of these SEDDS for preparations containing 5mg of Indapamide, which was a standard dose for the marketed products.
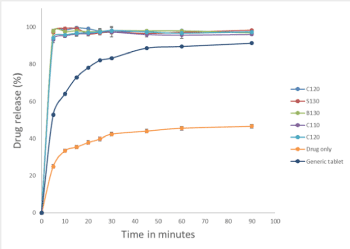
Figure 4. Dissolution profiles of fresh SEDDS, unprocessed drug powder and marketed generic tablets using water as dissolution medium. Values were represented as mean and standard deviation
Stability profiles of SEDDS were observed for up to 6 months under normal storage condition (25oC) and at high temperature (40ºC). The dissolution profiles of SEDDS at 3- and 6-months exhibited same patterns and they were not significantly different when compared to fresh samples when stored at 25oC (p>0.05) (Figure S2). At 40oC, similar pattern was observed except for SEDDS with 10% Solutol®HS15 (ID=S130) where the overall release was reduced to 91% possibly due to limited re-precipitation. Thus, dissolution enhancement by SEDDS was not compromised for most SEDDS. When comparing size distribution of the stored SEDDS, they were similar to their respective fresh samples, for instance, SEDDS with 10% Solutol®HS15 with the smallest size and SEDDS without the third component gave the highest PDI regardless of the storage temperatures (Figure S3&S4). Nonetheless, stored samples tend to form smaller droplets without affecting PDI patterns (p>0.05) partly due to aging or heat exposure. Hence, the stored lipid-based systems maintained their self-emulsifying capacity.
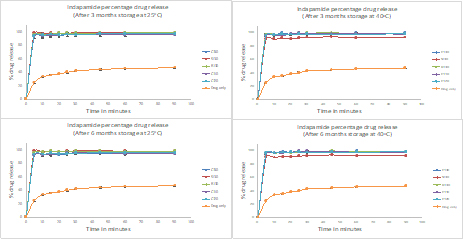
Figure S2. Dissolution profiles of SEDDS from stability study using water as dissolution medium (n=3). Values were represented as mean and Standard deviation. Unprocessed drug powder was used as control.
Top left: Indapamide release for lipid-based systems stored at 25ºC for 3-months.
Bottom left: Indapamide release for lipid-based systems stored at 25ºC for 6-months.
Top right: Indapamide release for lipid-based systems stored at 40ºC for 3-months.
Bottom right: Indapamide release for lipid-based systems stored at 40ºC for 6-months.
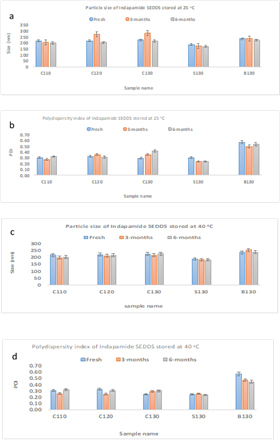
Figure S3. Size distribution of SEDD at 0, 3- and 6-months. Values were represented as mean and standard deviation (n=3). Droplet size of fresh and stored SEDDS stored at (a) 25ºC and (c) 40ºC. Polydispersity index (PDI) of fresh and stored SEDDS stored at (b) 25ºC and (d) 40ºC
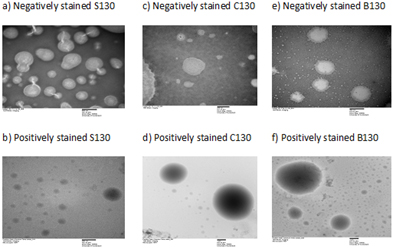
Figure S4. Images of S£DDS from transmission electron microscopy. (a) S130 negatively stained at 40000x; (b) S130 positively stained at 30000x; (c) C130 negatively stained at 70000x; (d) C130 positively stained at 10000x; (e) B130 negatively stained at 40000x; (f) B130 positively stained at 10000x
In this study, lipid-based system consisting of Labrasol, Labrafil®M1944CS and Capryol™90 has been considered as the best preparation as it can self-emulsify into SEDDS with small size distribution and with significantly enhanced Indapamide dissolution at the smallest drug to lipid ratio. Moreover, independent of drug to lipid ratios, the SEDDS remains stable for up to six months regardless of storage conditions. The dissolution enhancement was resulted from the ability of this lipid-based system to self-emulsify with formation of stable nano-sized dispersions, high drug solubility in SEDDS and low CMC of the dispersed system to withstand dilution by dissolution medium.
All the authors of this manuscript report no declaration of conflict of interest.
- Hauss DJ (2007) Oral lipid-based Formulations. Adv Drug Deliv Rev 59: 667-676. [Crossref]
- Vithlani S, Sarraf S, Chaw CS (2012) Formulation and in vitro evaluation of self-emulsifying formulations of Cinnarizine. Drug Dev Ind Pharm 38: 1188-1194. [Crossref]
- Saleh A, McGarry K, Chaw CS, Elkordy AA (2018) Feasibility of Using Gluconolactone, Trehalose and Hydroxy-Propyl Gamma Cyclodextrin to Enhance Bendroflumethiazide Dissolution Using Lyophilisation and Physical Mixing Techniques. Pharmaceutics 10: 22. [Crossref]
- Savjani KT, Gajjar AK, Savjani JK (2012) Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm 2012: 195727. [Crossref]
- Rutsaert R, Fernandes M (1983) Indapamide New Drugs Annual: Cardiovascular Drugs,edited by Alexander Scriabine. 45-67.
- Stetinova V, Kubant P, SvobodaZ, Kopecky J, Smetanova L, et al. (2007) In vitro and in vivo prediction of indapamide gastrointestinal absorption. Toxicology Letters 172: S56. [Crossref]
- Bremmell KE, Prestidge CA (2019) Enhancing oral bioavailability of poorly soluble drugs with mesoporous silica-based systems: opportunities and challenges. Drug Dev Ind Pharm 45: 349-358. [Crossref]
- Edueng K, Mahlin D, Larsson P, Bergströma CAS (2017) Mechanism-based selection of stabilization strategy for amorphous formulations: Insights into crystallization pathways. J Control Release 256: 193-202. [Crossref]
- Knapik J, Wojnarowska Z, Grzybowska K, Jurkiewicz K, Tajber L, et al. (2015) Molecular Dynamics and Physical Stability of Coamorphous Ezetimib and Indapamide Mixtures. Mol Pharm 12: 3610-3619. [Crossref]
- Wojnarowska Z, Grzybowska K, Hawelek L, Dulski M, Wrzalik R, et al. (2013) Molecular Dynamics, Physical Stability and Solubility Advantage from Amorphous Indapamide Drug. Mol Pharm 10: 3612-3627. [Crossref]
- Feeney OM, Crum MF, McEvoy CL, Trevaskis NL, Williams HD, et al. (2016) 50years of oral lipid-based formulations: Provenance, progress and future perspectives. Adv Drug Deliv Rev 101: 167-194. [Crossref]
- Balakrishnan P, Lee BJ, Oh DH, Kim JO, Lee YI, et al. (2009) Enhanced oral bioavailability of Coenzyme Q10 by self-emulsifying drug delivery systems. Int J Pharm 374: 66-72. [Crossref]
- Yeo LK, Olusanya TOB, CS Chaw, Elkordy AA (2018) Brief Effect of a Small Hydrophobic Drug (Cinnarizine) on the Physicochemical Characterisation of Niosomes Produced by Thin-Film Hydration and Microfluidic Methods. Pharmaceutics 10: 185. [Crossref]
- Bello V, Mattei G, Mazzoldi P, Vivenza N, Gasco P, et al. (2010) Transmission electron microscopy of lipid vesicles for drug delivery: comparison between positive and negative staining. Microsc Microanal 16: 456-461. [Crossref]
- Naved A, Rub MA, Asiri AM, Bawazeer WA (2017) Micellar and interfacial properties of amphiphilic drug‑non‑ionic surfactants mixed systems: Surface tension, fluorescence and UV‑vis studies. Colloid Surf Physicochem Eng Asp 522: 183-192. [Crossref]
- Mahmoud DB, Shukr MH, Bendas ER (2014) In vitro and in vivo evaluation of self-nanoemulsifying drug delivery systems of cilostazol for oral and parenteral administration. Int J Pharm 476: 60-69. [Crossref]
- Brouwers J, Brewster ME, Augustijns P (2009) Supersaturating drug delivery systems: the answer to solubility-limited oral bioavailability? J Pharm Sci 98: 2549-2572. [Crossref]
- Anby MU, Williams HD, McIntosh M, Benameur H, Edwards GA, et al. (2012) Lipid Digestion as a Trigger for Supersaturation: Evaluation of the Impact of Supersaturation Stabilization on the in Vitro and in Vivo Performance of Self-Emulsifying Drug Delivery Systems. Mol Pharmaceutics 9: 2063-2079. [Crossref]
- Dai WG, Dong LC, Creasey AA (2011) Inhibiting the precipitation of poorly water-soluble drugs from Labrasol formulations. Pharm Technol 35: 50-54.
- Khames A (2017) Investigation of the effect of solubility increase at the main absorption site on bioavailability of BCS class II drug (risperidone) using liquisolid technique. Drug Deliv 24: 328-338. [Crossref]








