Abstract
Everyone has become painfully aware that women (and men) with a breast cancer gene(s) have an increased “potential” to develop breast cancer. These same individuals also have an increased risk for developing uterine and ovarian cancer and in men, there is an increased potential to develop prostate cancer. A friend of mine, Dr. Henry Lynch is the man who discovered the breast cancer genes. He found two such genes in families whose women had multiple breast cancers. He called these genes BRCA1 and BRCA2. BR is the abbreviation for “breast” and CA is the abbreviation for “cancer.” The first one he discovered was numbered 1 and the second gene number 2. These genes are found on chromosomes 17 and 13 respectively. Because these genes increase the carriers “potential” for several different types of cancer, each of which are hormonally mediated, the primary author refers to these genes as “hormonally” mediated cancer genes.
Key words
breast cancer, cancer genes, mutation, Mammography
The function of the “normal” genes is to “suppress”, “inhibit” or “stop” cancers from developing in the first place. In the case of BRCA1 and BRCA2, these genes have become “altered” and no longer do their job. As a result, the genes don’t work properly to “prevent” cancer and if the person for whatever reason develops breast cancer, the “normal” genes won’t be there to stop it. So having BRCA1 and/or BRCA2 doesn’t actually cause cancer, they merely remove one of your safety mechanisms for trying to stop the development of cancer.
Some groups of people have a greater tendency to have these mutations. For example, Ashkenazi Jews have a 1 in 40 frequency of having one of these two mutations. The genes are seen less frequently in the U.S. population occurring once in every 400-800 people. Despite the number of people who have these “abnormal” genes, it is still only present in 5 to 10% of all breast cancer cases.
One of the very first reactions women have when they are told they have a BRCA1/2 gene is “I want them removed” and by them, they mean their breasts. The procedure is called a “prophylactic bilateral mastectomy” except there is nothing “prophylactic” about this radical surgery. We all know or have heard about people including celebrities like Angelina Jolie, who have undergone the radical removal of their breasts once they found out they had one or both of these genes, or one of any of a number of genes which may “predispose” them to cancer. Many people out of fear and quite frankly the inability of physicians to overcome the 35% error rate associated with Mammography and other testing so commonly used today.
Absent an accurate “quantitative” method for detecting breast cancer, one can understand women wanting something done to reduce their risk of dying from breast cancer. Women, who opt for bilateral prophylactic mastectomies, or mastectomy when cancer is present in their breast tissue, believe the mastectomy will totally remove their risk of developing breast cancer in the future. Unfortunately, mastectomy does not remove all breast tissue as there remains some residual breast tissue embedded in the skin, which resided over the now absent breast. Once a woman has undergone a mastectomy, then like men who can also develop breast cancer, there is less breast tissue remaining, but there is breast. Under these circumstances, if breast cancer develops in the remaining breast tissue, that cancer will have less breast tissue to grow through before reaching the chest wall and metastasizing through the lymphatics to the lung, liver, bones and brain as well as the rest of the body.
The real problem is one of uncertainty! Our current diagnostic tests are wrong 35% of the time, so the fear for women and men who have these genes is completely understandable. Fear of developing breast cancer, which can kill you, coupled with the erroneous perception that a bilateral (prophylactic) mastectomy somehow removes this risk, have fueled chaos and potentially worse outcomes if and when a breast cancer does actually develop. This is a classic example of two wrongs don’t make a right.
Nothing about mammography has resulted in anyone being so confident in its ability to detect breast cancer that we as physicians are willing to tell you that we can find breast cancer early enough to guarantee your survival. So, fear can easily influence patient decisions and if I were in the place of a woman with a breast cancer gene, I’m not certain I wouldn’t do the same thing. The only thing stronger than Fear is Hope!
Fortunately it’s 2018 and this is not the end of the story. The Fleming Method for Tissue and Vascular Differentiation-Breast Enhanced Scintigraphy Test (FMTVDM-BEST©®℗) or Breast Enhanced Scintigraphy Test (B.E.S.T.)©? Imaging recently received its patent approval after being studied in more than 1000 women and men [1-8]. FMTVDM-BEST©®℗ looks for breast cancer in an entirely different way. It was designed to use the characteristics of cancers to make them more easily detected and can accurately “quantify” or “measure” the differences between cancer and other types of tissue.
The method (Figure 1) briefly “enhances” regional blood flow and metabolic differences between calcium, normal breast tissue, inflammatory changes in the breast, pre-cancers and breast cancer and then measures these differences, allowing (1) differentiation of tissue types, (2) analysis of how rapidly the breast tissue is changing and (3) a determination of whether treatment is working or not.
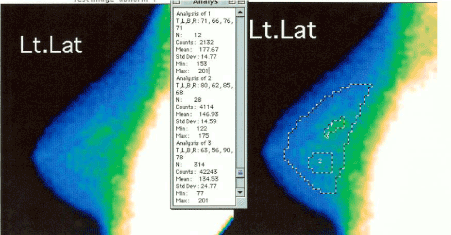
Figure 1. “Quantitative” analysis of FMTVDM-BEST©®℗ Imaging results.
Following “enhanced” image acquisition, breasts undergo independent “quantification” using FMTVDM-BEST©®℗ to define tissue differentiation. Here the initial “qualitative” image of a woman’s left breast in the left panel undergoes further “quantification” showing “inflammatory” to “pre-cancerous” changes in both regions 1 and 2. Region 1 demonstrates early Ductal Carcinoma In-situ (DCIS) changes.
As a result FMTVDM-BEST©®℗ can accurately find cancers and pre-cancers (Figure 2) missed by mammography and other tests. Because FMTVDM-BEST©®℗ is a quantitative test and does not rely on physician interpretation of what s/he sees on the image, it is not plagued by tissue density, clinical interpretation, calcium deposits, or even breast implants.
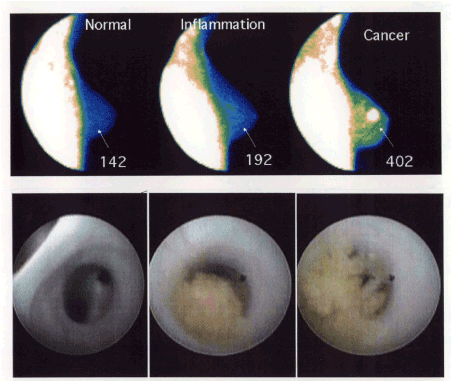
Figure 2. FMTVDM-BEST©®℗ results on three different women [3].
Three different women underwent FMTVDM-BEST©®℗ Breast Cancer Imaging followed by ductoscopy. The “quantification” of these “enhanced” BEST©? Images revealed “normal” (left panel), “late inflammatory-DCIS” (middle panel) and “cancer” (right panel) with the corresponding ductoscopy results shown in the panels below each.
As shown in Figure 3, FMTVDM-BEST©®℗ imaging revealed that despite this woman having previously had a mastectomy in addition to chemotherapy, she continued to have recurrent breast cancer. This example reveals just how easily FMTVDM-BEST©®℗ imaging can find breast cancers where mammography cannot and how FMTVDM-BEST©®℗ can be used to monitor treatment response, allowing for patient tailored treatment decisions, improving clinical outcomes and machine learning without physician error.
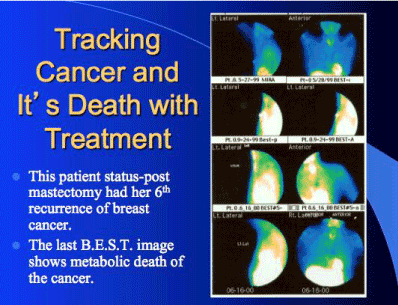
Figure 3. Patient monitoring of treatment response of her sixth breast cancer recurrence.
Over the course of 1-year, this woman underwent bilateral mastectomy, chemotherapy, radiation therapy, and diet and lifestyle changes. The first two rows show the recurrence of breast cancer in the left breast. Rows three and four finally show successful treatment. The “quantitative” values are not shown in this example.
The results for this patient in Figure 3 clearly shows that mastectomy itself could not, cannot and did not guarantee that breast cancer would not develop following the mastectomy. If mastectomy could guarantee this, then there would be no reason for a follow up visit with your doctor after the surgery. Since people can develop recurrent breast cancer after mastectomy, clearly a bilateral prophylactic mastectomy for individuals with a breast cancer gene(s) will not prevent breast cancer from developing?
As Figure 4 shows, FMTVDM-BEST©®℗ imaging can even be used even for women who have breast implants, a relatively common procedure for those who have undergone mastectomy, either for their BRCA1/2 genes “prophylactic” surgery or for the actual treatment of breast cancer itself.
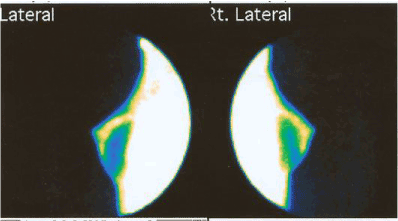
Figure 4. FMTVDM-BEST©®℗ Breast Cancer Imaging following bilateral mastectomy and breast implants.
Following a bilateral mastectomy, this woman elected to have breast implants. As can be seen, there is residual breast tissue in the upper and anterior regions of both breasts. The “enhanced” results also demonstrate that FMTVDM-BEST©®℗ Imaging easily distinguishes between the native residual breast tissue and the breast implants. The “quantified” results are not included here for purposes of illustrating the above noted differences.
While genetic testing may be helpful in making life-changing decisions, our prior inability to more accurately detect breast cancer, has undoubtedly caused many women (and men) to make drastic decisions based upon fear; fear that we in Medicine have not been able to previously adequately address given the inaccuracy of our previously qualitatively driven tests.
Conclusion
FMTVDM-BEST©®℗ imaging is the First quantitative method which changes that Fear; providing the physician and patient, the person, including those with prior breast cancer and those with a genetic predisposition toward breast caner, the additional information we NEED before deciding on a treatment plan, including surgery, chemotherapy, hormonal therapy, dietary and lifestyle changes, or nothing at all.
Acknowledgements
This study is funded by the Camelot Foundation 501(c)(3). All authors reviewed and approved of manuscript submission.
Conflicts of interest
Primary author has been issued the patent and copyrights used in this article.
Copyright
2021 Copyright OAT. All rights reserv
FMTVDM-BEST©®℗ Protected. The primary author has authorized the reproduction and use of terms for purposes of this publication only.
References
- Fleming RM (2002) Breast enhanced scintigraphy test demonstrates improvement in breast inflammation in women consuming soy protein. J Nutr 132: 575S.
- Fleming RM (2002) Mitochondrial Uptake of Sestamibi Distinguishes Between Normal, Inflammatory Breast Changes, Pre-cancers and Infiltrating Breast Cancer. Integr Cancer Ther 1: 229-237. [Crossref]
- Fleming RM, Dooley WC (2002) Breast Enhanced Scintigraphy Testing (B.E.S.T.) Distinguishes Between Normal, Inflammatory Breast Changes and Breast Cancer. A Prospective Analysis and Comparison with Mammography. Integr Cancer Ther 1: 238-245. [Crossref]
- Fleming RM (2003) What effect, if any, does soy protein have on breast tissue? Integr Cancer Ther 2: 225-228. [Crossref]
- Fleming RM (2003) Are there differences in breast tissue as a result of hormone replacement therapy? Can BEST imaging distinguish these differences? Integr Cancer Ther 2: 229-234. [Crossref]
- Fleming RM (2003) Do women taking hormone replacement therapy (HRT) have a higher incidence of breast cancer than women who do not? Integr Cancer Ther 2: 235-237. [Crossref]
- Fleming RM (2004) Breast Enhanced Scintigraphy Test demonstrates improvement in breast disease after daily consumption of soy protein. J Nutr.
- Fleming RM, Dooley WC, Chaudhuri TK (2017) The Development of FMTVDM-BEST IMAGING©?: The Answer for Breast Cancer. Breast Enhanced Scintigraphy Test (BEST©?): Quantifying the Detection of Breast Cancer and its Treatment. J Nucl Med Radiat Ther 8: 350.




