Objective: Effect on blood pressure and safety of Garcinia kola seed supplementation in patients with high normal blood pressure (BP) or grade I hypertension (HTN).
Methods:
Design, setting and participants: A single center, single arm non – randomized clinical trial in patients with high normal BP or grade I HTN conducted between January and May 2019 in one teaching hospital in Cameroon. Blood pressure was measured using Ambulatory Blood Pressure Monitoring (ABPM) before and after the intervention. Participants were 25 adult patients with high normal BP or grade I HTN newly diagnosed and naïve to any antihypertensive treatment.
Intervention: Daily supplementation with 2 seeds (10 grams each) of Garcinia kola. Follow-up 30 days.
Main outcome measure: Absolute change from baseline to final visit in median BP.
Results: Twenty-four participants were analyzed (median age 56 [41–62] years). One participant was lost to follow-up. The median blood pressure at Day 30 vs. baseline was significantly lower (123 mmHg vs. 136 mmHg, difference – 13 mmHg [95% CI, – 10 to – 18 mmHg], p<0.01; and 76 mmHg vs. 84 mmHg, difference – 7 mmHg [95% CI, – 4 to – 13 mmHg], p<0.01; respectively for systolic and diastolic blood pressure). No serious adverse effect was observed.
Conclusion: Garcinia kola seed supplementation in patients with high normal or grade I hypertension is safe and reduces significantly their BP.
Garcinia kola; supplementation, hypertension
The number of people living with hypertension (HTN) worldwide was estimated at 1.13 billion in 2015 [1] with a prevalence in adults spanning from 30 to 45% [2]. According to some scientists, this number should increase by 15 to 20% in 2025 to reach 1.56 billion [3]. Furthermore, HTN is responsible of 17 million deaths and 200 million disabilities secondary to its complications which makes it a public health issue [4]. It is taking alarming proportions in Africa with prevalence rates higher than those of developed countries and estimated to 46% in adults aged 25 years or more according to the World Health Organization (WHO) [5]. In Cameroon, Kingue et al found a prevalence de 29.7% in urban areas in 2015 [6]. Given these high rates, emphasis should be made on the prevention and treatment of this important cardiovascular risk factor. Despite the efforts done, the fight against HTN is hindered by the high cost of medications. Approximately 80% of rural populations living in low-income countries rely of traditional medicine for their treatment [7]. We should therefore intensify research on medicinal plants which are thought to have anti-hypertensive properties to include them as treatment modalities [8]. Many medicinal plants used for the treatment of HTN have been identified during ethnobotanical surveys. One of them is Garcinia kola which is used in Africa, and in Cameroon in particular, for the treatment of many diseases. Locally called (bitter kola), Garcinia kola is a medicinal plant having many properties. It contains substances that give it antibacterial, antifungal, antiulcer, anti-inflammatory, antidiabetic, and anti-hypertensive properties [9]. A study done on rats has shown anti-hypertensive action of Garcinia kola [10] but to our knowledge, its efficacy has never been tested in humans before. We therefore aimed to evaluate the effect on BP and safety of Garcinia kola seed supplementation in some patients at the Yaoundé Central Hospital (YCH).
Study design, setting and participants: We conducted a single center, single arm non-randomized clinical trial from January to June 2019. Participants were recruited and data was collected at the cardiology unit of the YCH. All patients, 18 years of age or older, with HTN who presented for consultation were screened for eligibility to the trial. Inclusion criteria were high normal blood pressure or grade I HTN as defined by the WHO and no previous anti-hypertensive treatment. Exclusion criteria included consumption of Garcinia kola seeds or other medicinal plants during the two weeks preceding enrollment in the trial, creatinine clearance lower than 60ml/min/1.73 m2 or history of chronic kidney disease, alanine transaminase (ALT) level higher than three times the normal value and lost to follow-up. Patients with diabetes mellitus were not excluded from this trial. An informed consent was obtained from all the participants. The trial was approved by the Centre region ethical review board and all ethical principles for medical research involving human subjects stated in the Declaration of Helsinki were respected.
Intervention
The Garcinia kola seeds we used in this trial were authenticated at the national herbarium of Cameroon and were weighted then packaged according to the daily dose to be taken by each participant. A run-in phase of two weeks was observed before enrollment during which there was repeated measurements of office blood pressure. Demographic, background, and pathologic data were obtained through a questionnaire and review of medical records. Anthropometric measurement, ABPM and laboratory assessments were conducted at enrollment and after 30 days of intervention. All Participants were asked to eat two seeds of Garcinia kola weighting 10 ± 0.2 g each, every morning before breakfast at 8 AM ± 1 hour for a total of 4 weeks. They were contacted by telephone once weekly to document supplement adherence and to monitor for the development of adverse effects. At 4 weeks of supplementation, patients then returned for a follow-up visit for follow-up ABPM and laboratory measurements.
Outcomes
The primary endpoint was the absolute change from baseline to final visit in blood pressure. The secondary endpoint was the absolute change from baseline to final visit in lipid profile parameters. The effect of Garcinia kola seed supplementation on glycaemia was not a predefined endpoint in this trial. All end points were evaluated in the primary analysis population, defined as all enrolled patients who had measurements at both baseline and final visit. Adverse effects were recorded from enrollment to 4 weeks of Garcinia kola seed supplementation.
Study Measurements
Ambulatory blood pressure monitoring: A 24-hour ABPM was performed for all participants at enrollment and at 4 weeks. It was done using an electronic blood pressure machine of the CONTEC® brand.
Laboratory measurements: All laboratory measurements were carried out at enrollment and at 4 weeks. They were conducted in the laboratory of the national center of obesity.
Security of the trial and monitoring of adherence to treatment: Participants were contacted by telephone once weekly to document supplement adherence and palatability, and to monitor for the development of adverse effects. Adherence to the treatment was evaluated using Girerd compliance evaluation test which is a modified version of the Morisky medication adherence scale.
Statistical Methods
Data were analyzed using Statistical Package for Social Sciences (SPSS®) software version 23.0 for Windows®. Data were expressed as frequencies and percentages for categorical variables and as median and interquartile range for continuous variables. The change in BP and lipid profile parameters was defined as the value post-supplementation minus the value pre-supplementation. The difference between two skewed continuous variables was compared using the Wilcoxon signed-rank test. All p values < 0.05 were considered statistically significant.
The flow of participants at each stage of the study is shown in Figure 1. Participants enrolled in the trial were 25. 1 was lost of follow-up and 24 were analyzed. Men (62.5%) were more represented than women and the median age was 56 [41–62] years.
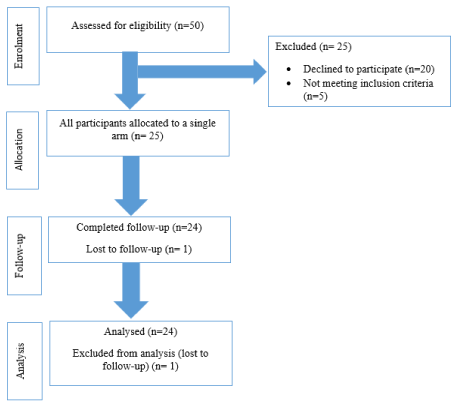
Figure 1. Flow of participants through each stage of the study.
Table 1 shows the clinical and laboratory measurements of the participants before and after intervention. The blood levels of creatinine and alanine aminotransferase were normal at enrollment and remained unchanged at the end of the trial. There was a significant change of 24h blood pressure and lipid profile parameters after 4 weeks Garcinia kola seed supplementation compared to baseline. These changes are shown in Figures 2 and 3. We had a median change was – 13 mmHg ([95% CI, – 10 to – 18 mmHg], p<0.01) for the 24h systolic blood pressure and – 7 mmHg ([95% CI, – 4 to – 13 mmHg], p<0.01) for diastolic blood pressure. For the lipid profile parameters, the median changes were – 0.080 g/l ([95% CI, – 0.070 to – 0.100], p<0.01), – 0.065 g/l ([95% CI, – 0.045 to – 0.090], p<0.01), – 0.090 g/l ([95% CI, – 0.070 to – 0.110], p<0.01) and +0,085 g/l ([95% CI, +0.080 to +0.090], p<0.01) for respectively total cholesterol, LDL cholesterol, triglycerides and HDL cholesterol.
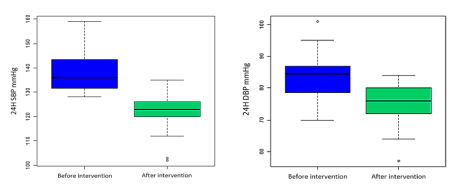
Figure 2. Change of 24 hour systolic and diastolic blood pressure before and after intervention.
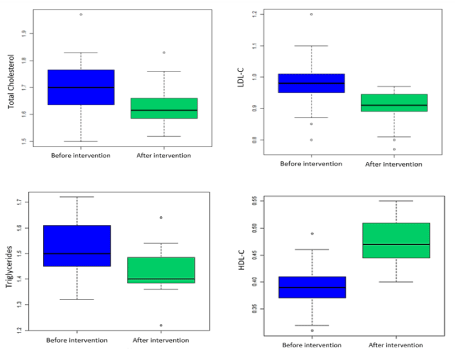
Figure 3. Change of lipid profile parameters before and after intervention.
Table 1. Clinical and biological characteristics of participants at baseline and after intervention.
Parameters |
Before intervention
Median [IQI]
N=24 |
After intervention Median [IQI]
N=24 |
p |
Weight (kg) |
92.0 [71.0 – 101.0] |
91.5 [71.2 – 101.5] |
0.7 |
BMI (kg/m2) |
31.7 [25.4 – 34.8] |
31.4 [25.3 – 34.3] |
0.48 |
Waist /Hip circumference |
0.94 [0.91 – 0.96] |
0.93 [0.89 – 0.96] |
0.9 |
Resting SBP (mmHg) |
145 [136 – 153] |
128 [125 – 130] |
< 0.01 |
Resting DBP (mmHg) |
92 [90 – 96] |
83 [81 – 85] |
< 0.01 |
Heart rate (bpm) |
81 [75 – 88] |
82 [78 – 85] |
0.6 |
Glycaemia (g/l) |
1.4 [1.2 – 1.7] |
1.3 [1.3 – 1.6] |
0.72 |
ALAT(UI/l) |
27.2 [27.0 – 29.0] |
27.0 [26.0 – 28.7] |
0.75 |
Creatininemia (mg/l) |
8.0 [7.5 – 8.9] |
8.2 [7.8 – 8.5] |
0.73 |
Triglycerides (g/l) |
1.5 [1.4 – 1.6] |
1.4 [1.3 – 1.4] |
< 0.01 |
Total cholesterol (g/l) |
1.7 [1.6 – 1.7] |
1.6 [1.5 – 1.7] |
< 0.01 |
HDL-cholesterol (g/l) |
0.39 [0.37 – 0.41] |
0.47 [0.44 – 0.51] |
< 0.01 |
LDL-cholesterol (g/l) |
0.98 [0.95 – 1.01] |
0.91 [0.89 – 0.94] |
< 0.01 |
BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; ALAT: alanine-amino-transferase; HDL: high density lipoprotein; LDL: low density lipoprotein
More than half of the participants (54.2%) answered that the Garcinia kola seeds had a bad taste, but it did not interfere with the supplementation. There was a 91.7% rate of good compliance and 8.3% rate of minor noncompliance to the intervention on the Girerd compliance evaluation test. Tree (03) adverse effects were reported during the monitoring of the intervention. These were one case of intermittent dizziness, one case of agueusia and one case of nausea.
We conducted this trial to assess the effect of Garcinia kola seeds on the 24-hour BP in patients with high normal BP and grade I HTN. Our study has the advantage of being the first to be done in humans, the blood pressure was monitored using 24h ambulatory blood pressure monitoring. However, there were some limitations that have to be addressed especially the Hawthorne effect that can be a confounding factor leading to an overestimation of the effectiveness of our intervention [11].
Naiho et al., who worked on murine models of high blood pressure in Benin City also found a statistically significant reduction of the systolic blood pressure with a mean reduction of 19 mmHg after three weeks of feeding with Garcinia kola seeds [10]. The authors hypothesized that the anti-hypertensive effect of Garcinia kola seeds may be mediated by the reduction of peripheral vascular resistances. While verifying this hypothesis, Adaramoye et al. showed that kolaviron, a biflavonoid extract from the seeds of Garcinia kola, was responsible of vasodilation of mesenteric arteries of rats by the blockade of calcium influx in the vascular smooth muscle cells [12].
Uche et al also showed that kolaviron prevented the appearance of high blood pressure and improved the lipid profile of rats. They tried to induce high blood pressure in the rats by giving them high salt diet and compared the blood pressure between a group which received Garcinia kola seeds and another which did not. The mean blood pressure was 120/70 mmHg for the group receiving the seeds versus 170/110 mmHg for the control group. There was a similar finding concerning the lipid profile [13].
In another study, Adaramoye et al found the beneficial effect of kolaviron in rats with induced hypercholesterolemia [14]. The observed a 70% and 88% reduction of respectively LDL-cholesterol and total cholesterol after 8 weeks of treatment with Garcinia kola seeds. These levels of reduction were far higher than the ones we observed in our study. This may be explained on one hand by the shorter duration of our intervention which was 4 weeks only, and on the other hand by the fact that the rats in Adaramoye et al study had major hypercholesterolemia unlike our participants who had cholesterol levels close to the normal range. Adejor et al also found a significant reduction of the levels of total cholesterol, LDL-cholesterol and triglycerides, and an increase of HDL-cholesterol level of hyperlipidemic rats after administration of biflavonoid extracts of Garcinia kola seeds [15]. They had many groups of rats including one group treated with atorvastatin. The level of improvement of the lipid profile was similar in the groups treated with atorvastatin and Garcinia kola.
Throughout our experiment the level of transaminases and creatinine stayed unchanged. This indicates a good hepatic and renal tolerance of Garcinia kola seeds. No serious side effect was reported. Most of the participants answered that the seeds had a bad taste. This suggest that to use it as an alternative treatment of high blood pressure, the Garcinia kola seeds need to be processed to improve the taste and ease the consumption.
We succeeded in implementing our intervention has planned with little or no difficulty because of the fact that Garcinia kola seeds are already known widely consumed in the Cameroonian population. The results of this study can only be applied to people suffering from grade I hypertension with regards to the sample population. To determine if the use of Garcinia kola can be generalized to all hypertensive patients, more research need to be done.
Garcinia kola seed supplementation seem to have significant anti-hypertensive effects in patients with high normal and grade I HTN. It may also help to improve the lipid profile of the consumers. It is well tolerated with the only inconvenience of having a bad taste. This trial opens the way for further studies which can lead to a large-scale use of this medicinal plant for the treatment of high blood pressure in countries where there is limited access to high-cost modern drugs.
Ethics approval and consent to participate: The study was approved by the Centre Regional Ethics Committee for Human Health Research under the number CE N°1533/CRERSHC/2018. All participants gave a written consent at enrollment.
Consent for publication: All participants agreed to consent of publication.
Availability of data and material: The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Funding: No external funding was acquired for this research.
Conflict of interests: There is no conflict of interest.
Author’s contributions: LMK, NKC, DD and ES searched the literature and collected the data; RCNK, DD, LMK and ES performed the statistical analyses, and wrote the manuscript; LMK, PCD, EN and ES contributed to conception, design, data interpretation, and supervision of the study. All authors read and approved the final manuscript.
Acknowledgements: Thanks to the Yaoundé Central Hospital for supporting this trial with the necessary setting and material. Thanks also to all subjects who participated in this study.
- Zhou B, Bentham J, Di Cesare M, Bixby H, Danaei G, et al. (2017) Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. The Lancet 389: 37-55. [Crossref]
- Chow CK, Teo KK, Rangarajan S, Islam S, Gupta R, et al. (2013) Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA 310: 959-968. [Crossref]
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, et al. (2005) Global burden of hypertension: analysis of worldwide data. The Lancet 365: 217-223. [Crossref]
- Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, et al. (2017) Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990-2015. JAMA 317: 165-182. [Crossref]
- Nulu S, Aronow WS, Frishman WH (2016) Hypertension in Sub-Saharan Africa: A Contextual View of Patterns of Disease, Best Management, and Systems Issues. Cardiol Rev 24: 30-40. [Crossref]
- Kingue S, Ngoe CN, Menanga AP, Jingi AM, Noubiap JJN, et al. (2015) Prevalence and Risk Factors of Hypertension in Urban Areas of Cameroon: A Nationwide Population-Based Cross-Sectional Study. J Clin Hypertens 17: 819-824. [Crossref]
- Kofi-Tsekpo M (2021) Institutionalization of African Traditional Medicine in Health Care Systems in Africa. Afr J Health Sci 11: 1-2. [Crossref]
- Ngcobo M, Nkala B, Moodley I, Gqaleni N (2011) Recommendations for the Development of Regulatory Guidelines for Registration of Traditional Medicines in South Africa. Afr J Tradit Complement Altern Med 9: 59-66. [Crossref]
- Buba CI, Okhale SE, Muazzam I (2016) Garcinia kola: The phytochemistry, pharmacology and therapeutic applications. Int J Pharm Sci Res 3: 67-81.
- Naiho AO, Ugwu AC (2009) Blood pressure reducing effect of bitter kola (garcinia kola, heckel) in wistar rats. Afr J Biomed Res 12: 131-134.
- Grimshaw J, Campbell M, Eccless M, Steen N (2000) Experimental and quasi-experimental designs for evaluating guideline implementation strategies. Fam Pract 17: 11-16. [Crossref]
- Adaramoye OA, Medeiros IA (2009) Endothelium-independent vasodilation induced by kolaviron, a biflavonoid complex from Garcinia kola seeds, in rat superior mesenteric arteries. J Smooth Muscle Res 45: 39-53. [Crossref]
- Uche OK, Osakpolor FA (2018) Kolaviron Attenuates Elevation in Blood Pressure and Ameliorates Dyslipidemia in Salt-Induced Hypertensive Sprague-Dawley Rats. Afr J Biomed Res 21: 219-224.
- Adaramoye OA, Nwaneri VO, Anyanwu KC, Farombi EO, Emerole GO (2005) Possible anti-atherogenic effect of kolaviron (a Garcinia kola seed extract) in hypercholesterolaemic rats. Clin Exp Pharmacol Physiol 32: 40-46. [Crossref]
- Adejor EB, Ameh DA, James DB, Owolabi OA, Ndidi US (2016) Effects of Garcinia kola biflavonoid fractions on serum lipid profile and kidney function parameters in hyperlipidemic rats. Clin Phytoscience 2: 19.
Editorial Information
Editor-in-Chief
Massimo Fioranelli
Guglielmo Marconi University
Article Type
Research Article
Publication history
Received: October 02, 2021
Accepted: November 26, 2021
Published: December 06, 2021
Copyright
©2021 Kuate LM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Kuate LM, Negue Kuate RC, Danwe D, Nnanga E, Menanga A, et al. (2021) Garcinia kola seed supplementation reduces the blood pressure in patients with high normal blood pressure or grade I hypertension: a single center, single arm non - randomized clinical trial. J Integr Cardiol. 7. DOI: 10.15761/JIC.1000310



