Background: Neurofibromas are rare benign tumors of neural origin, usually associated with Neurofibromatosis type 1, an autosomal dominantly inherited syndrome. However, they can also be solitary, and appear in the oral cavity. Gingival localization is rare and takes a hypertrophic form associated with an attachment loss that can mimic a plaque-induced- periodontal disease.
Case presentation: The patient was a 52-year-old female patient. She was mistakenly referred to our department for periodontal surgical debridement, whereas she presented a symptomatic gingival solitary neurofibroma. The surgical tumour excision allowed a good periodontal healing, and no recurrence was observed after 7 years.
Conclusions: The aim of this case report was to illustrate the essential role of the dental clinician in the detection of tumoral non plaque-induced gingival diseases. Knowledge of these tumor characteristics facilitates a more comprehensive differential diagnosis approach, thus avoiding improper management or late diagnosis.
gingival neurofibroma, neurofibromatosis type 1, non-plaque-induced gingivitis, case-report
NF1: neurofibromatosis type 1; EMA: epithelial membrane antigen antibody; MRI: magnetic resonance imaging
Neurofibroma is a benign peripheral nerve sheath tumor, generated by the proliferation of Schwann cells, perineural cells, and endoneural fibroblasts. It may occur either as a single lesion, called solitary neurofibroma, or as part of a generalized syndrome known as Neurofibromatosis type 1 (NF1), which is a systemic disorder caused by a mutation in the NF1 tumor suppressor gene located at 17q11.2; it is also known as von Recklinghausen’s disease. Without treatment, these lesions may generate serious functional complications, and even develop into malignant tumors.
Solitary neurofibroma is uncommon in the oral cavity, and only a few cases have been reported in the literature. Clinically, oral neurofibromas are soft, sessile, asymptomatic submucosal tumors which grow slowly, and may vary in size ranging from a small nodule to a large mass. They may occur at any site in numerous oral locations such as the tongue, the palate, the cheek mucosa, the floor of the mouth, or as intra-osseous lesions. The diagnosis is confirmed by histologic examination and immunohistochemistry, essentially based on the S-100 protein detection, indicating their neural origin.
This article reports the rare and successfully treated case of a symptomatic gingival solitary neurofibroma in a female patient free of any manifestation of Neurofibromatosis type 1. This case highlights the importance of differential diagnosis, which is crucial when managing such an atypical situation, and illustrates the key role of the dental clinician in detecting isolated gingival tumors.
In March 2012, a 52-year-old female patient was referred to the Department of Oral Surgery and Oral Pathology of the Henri Mondor Hospital. The patient was referred to us by her dentist for periodontal surgery on the right mandibular molars, due to a recurrent severe lingual and interdental attachment loss, despite prior non-surgical root planning and debridement. The patient’s chief complaint expressed during the first appointment was gingival swelling in the lower arch, slowly developing since November 2011, and associated with moderate and intermittent irradiating pain. The patient was a nonsmoker, and with no particular medical history.
Clinical examination revealed a localized and painless hypertrophy involving the marginal and attached lingual gingiva extending from the mesial aspect of the first right mandibular molar to the distal aspect of the second right mandibular molar. The lesion was renitent, non-ulcerated, sessile and with a normal colour (Figure 1). No lymphadenopathy was observed.
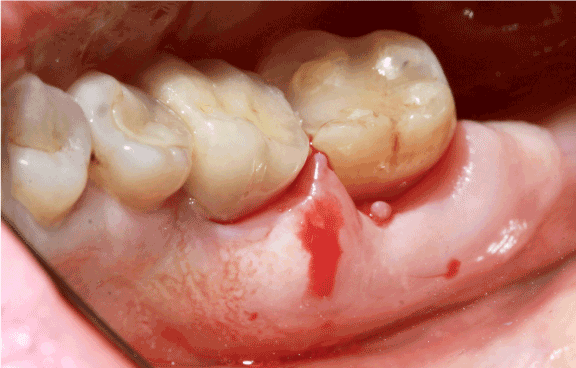
Figure 1. Localized lingual gingival hypertrophy. The lesion extended from the mesial aspect of tooth 46 to the posterior commissure
Tooth 46 had been crowned years ago. Dental pulp testing revealed no abnormal response for tooth 47; however, periodontal probing identified a severe clinical attachment loss (12 mm on tooth 46, 10 mm on tooth 47) on the lingual aspect, without any tooth mobility. The periapical radiographs showed a slight interdental alveolar bone loss with no soft tissue calcification (Figure 2). Despite an inadequate root canal filling on tooth 46, no clinical sign nor radiolucent image indicating a possible secondary endodontic infection (endo-perio lesion) was observed.
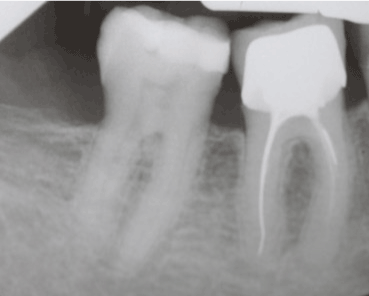
Figure 2. Periapical radiograph of the lower right first and second molars
Differential diagnosis
The unusual aspect of the periodontal lesion – severe clinical attachment loss without any endodontic infection, and associated with slight bone loss – was unlikely for a common plaque-induced periodontal involvement, and required a comprehensive differential diagnosis. From the very start, the general health status and the absence of medication excluded haematological malignancy and drug-induced gingival enlargement. Moreover, we excluded all the possible etiologies associated with generalized gingival hypertrophy, such as plaque-induced gingivitis exacerbated by a risk factor, plasma cell gingivitis, vitamin C deficiency, granulomatous disease or hereditary gingival fibromatosis.
Besides these diagnostic considerations, pyogenic granuloma, peripheral ossifying fibroma and peripheral giant cell granuloma were most unlikely as the gingiva showed a normal pink color and no nodular exophytic growth (Figure 1). Furthermore, absence of calculus or focal calcification on the periapical radiograph excluded pyogenic granuloma and peripheral ossifying fibroma as potential diagnostic considerations (Figure 2). Consequently, the hypothesis of a benign gingival tumor appeared as a more credible diagnosis.
Gingival biopsy was performed and sent for histopathological analysis. The gingival sample revealed a dense spindle-shaped cell proliferation within a fibrillar stroma, associated with capillary and mast cell proliferation, showing no plexiform aspect. Immunohistochemical staining was positive for S-100 protein, EMA (epithelial membrane antigen antibody) and smooth muscle actin (Figure 3). These results were consistent with a benign gingival neurofibroma; the localization indicated that the neurofibroma originated from the lingual nerve.
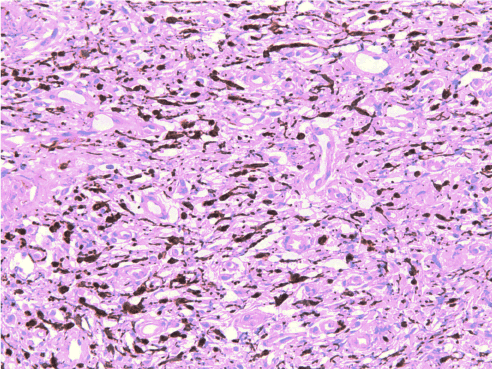
Figure 3. Immunostaining positive for S100 protein (x200). The tumor cells strongly express the S100 protein (intracytosolic calcium-binding protein), a biological marker of neurological pathologies
Magnetic resonance imaging (MRI) was then performed to exclude other tumor localizations, and to assess the local extent of the tumour. Detailed patient examination showed none of the following signs : café-au-lait spots, cutaneous neurofibroma, Lish nodule, optical pathway glioma, osseous lesion, thus excluding Neurofibromatosis type I, and allowing us to diagnose a solitary gingival neurofibroma on the lingual aspect of the right mandibular gingiva.
Treatment
The treatment consisted in surgical excision. Following patient information of a potential postoperative lingual nerve injury and her signed informed consent, the surgery was performed under local anaesthesia, using a 4% articaïne solution with 1/200000 epinephrine.The gingival lesion was carefully dissected and excised including surgical margins, using Metzenbaum scissors and a CO2 LASER (Figure 4). The surgical site was protected using collagen absorbable sponges and a periodontal dressing. The patient was sent home with a prescription of amoxicillin, prednisolone, acetaminophen + codeine, chlorhexidine mouth rinse and topical disinfectant. Postoperative healing was uneventful except for mild pain. No dysphagia or dysesthesia/paraesthesia was noted.
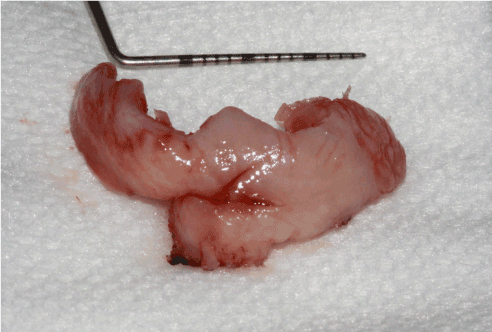
Figure 4. Postoperative surgical specimen of the lingual neurofibroma. In order to avoid post-operative sequelae, the neurofibroma was dissected in its apical portion (close to the floor of the mouth) with CO2 LASER (5 pulsed watts)
At 3 months follow-up, the lingual gingiva in the right mandibular region was completely healed, with no local inflammation or pain. 7-year follow-up revealed no sign of recurrence of the gingival neurofibroma (Figure 5).
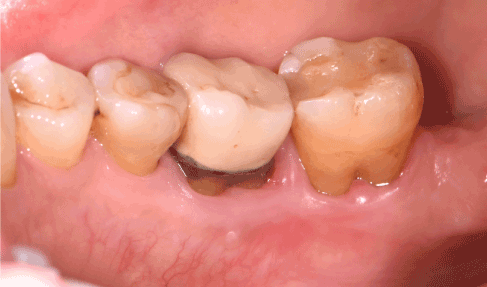
Figure 5. Healing of the lingual gingiva at 7 years follow-up. Periodontal recessions are stable, no inflammation or periodontal pocket is noticed
Neurofibromas are the most frequent benign neoplasms originating from the peripheral nerve sheath which may occur either as solitary or multiple tumors when related to Neurofibromatosis type 1 (NF1) [1-8]. The lesions most often appear on the skin; however, many organs can be involved including the stomach, intestines, kidneys, bladder, larynx and heart. Neurofibromas can be present at birth or develop anytime during life: they often affect adults and children ranging from 9 to 50 years of age, with a mean age of 31.2 years old [9-11]. Some studies have reported a female predominance [9,11-13] whereas other studies have shown no gender predominance for this lesion [10].
Based on clinical observation and medical history of the lesions, neurofibromas can be divided into two major categories, localized and plexiform [14]: localized neurofibromas which develop from a single site along a peripheral nerve., and depict a focal mass with well-defined margins; plexiform neurofibromas extend along a peripheral nerve and may involve multiple nerve branches [1,15]. They represent a major source of morbidity associated with NF1, mainly due to their tendency to grow and reach large sizes, thus causing disfigurement. The most involved cranial nerves in plexiform neurofibromas are the V, IX and X.
Neurofibromas are clinically characterized by slow growth, lack of pain, superficial location [16,17] and variable size [18] During puberty or pregnancy, their development may be accelerated due to intense hormonal disorders [19]. They are most often unilateral and nodular lesions [19].
Histologically, neurofibromas are non-encapsulated tumors formed by a complex proliferation of Schwann, perineural, endoneural fibroblastic and intermediate cells [1,3,16,17,20-22]. Subsequently, they present a large microscopic cell heterogeneity [4,12,13,23]. Generally, they are well-circumscribed tumors characterized by intermingled sheaves of elongated spindle-shaped cells with wavy or comma-shaped nuclei, within a myxoid matrix consisting of scattered delicate collagen fibers, and a variable number of mast cells [24]. In older lesions, hyaline changes may occur, although edematous changes are more likely, especially in the plexiform variety [25].
Histological examination alone is not sufficient for a final diagnosis of neurofibroma, which also requires immunohistochemistry. More specifically, a panel of immunoreactions including S100 protein, type IV collagen, CD34, EMA and neurofilament or neuron-specific tubulin (TUBB3) can be implemented [13].
Malignant transformation of neurofibromas has been a well-established phenomenon for decades [26,27], and their incidence is 3 to 5 % [1,28]. Identified as a malignant peripheral nerve sheath tumor, it often occurs in association with large diffuse neurofibromas, especially in NF1 patients [29]. Rapid enlargement of the neurofibroma and the presence of pain must suggest malignant transformation [14,30]. Once a malignant change has occurred, the lesions have a poor prognosis [31].
In the head and neck region, the most commonly affected sites are the scalp, cheek, neck and oral cavity. Nevertheless, oral neurofibromas remain quite uncommon [31,32], especially when not related to NF1 [11,31,33]: past literature reports that the frequency of solitary neurofibromas in the oral cavity is around 6.5%.
All the hard and soft oral tissues have been reported to be involved in these tumors [34]. However, the majority of oral neurofibromas occur in the tongue, oral mucosa and the lips [1,3,12,13,32]. Other less frequent sites include the gingiva [13,35] and the palate [11,36].
Their clinical aspect is not specific and does not much differ from cutaneous neurofibromas; solitary oral neurofibromas resemble localized nodules matching the color of the surrounding mucosa, and are usually asymptomatic [19,21,31,37]. The lesions are often diagnosed late due to their slow and painless growth, causing few symptoms [38]. However, when adjacent to cranial nerves, they can impair motor functions of facial/hypoglossal nerves or the sensitivity of trigeminal nerves [21,37]; they may even affect speech [39]. In case of gingival involvement, neurofibromas also cause tooth malposition or impaction [19].
Gingival neurofibromas are rare [37], most cases occurring in NF1 patients (5 % of cases) [40,41] (Figure 6). Within the limits of our research, few cases of solitary gingival neurofibromas have been fully described in the English literature (Table 1).
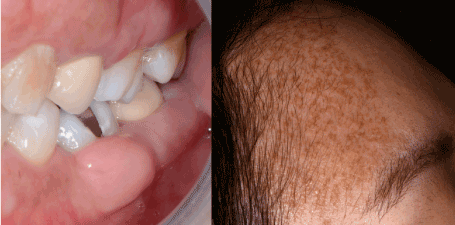
Figure 6. Gingival neurofibroma in the context of neurofibromatosis type I. The syndromic diagnosis was clinical, based on the presence of multiple cutaneous neurofibromas, cutaneous “café-au-lait” spots, and the history of a positive diagnosed mother. The diagnosis of gingival neurofibroma was confirmed by the biopsy
Table 1. Reported solitary oral neurofibromas in the literature
|
Gender |
Age |
Location |
Neurofibroma |
Diagnosis |
Treatment |
Recurrence |
Bruce [42] |
Male |
36 y.o. |
Mandibular posterior alveolar ridge |
Localized |
Biopsy |
Excision |
None after 6 months |
Richards [31] |
Female |
55 y.o. |
Buccal maxillary anterior gingival papilla |
Localized |
Biopsy |
Excision |
NS |
Alatli, et al. [43] |
Female |
37 y.o. |
Buccal mandibular posterior gingiva and mucosa |
Plexiform |
Biopsy |
Excision |
None after 2 years |
Sinha, et al. [44] |
Female |
50 y.o. |
Soft palate |
Localized |
Biopsy |
Excision |
None after 1,5 year |
Marocchio, et al. [45] |
Female |
24 y.o. |
Cheek mucosa |
Localized |
Biopsy
S-100 positivity |
Excision |
None after 3 years |
Depprich, et al. [21] |
Male |
64 y.o. |
Lingual mandibular gingiva and mouth floor |
Localized |
Biopsy
S-100 positivity |
Excision |
None after 1 year |
Ohno, et al. [46] |
Female |
32 y.o. |
Buccal mandibular posterior gingiva |
Localized |
Biopsy
S-100 positivity |
Excision |
None after 5 years |
Suramya, et al. [47] |
Female |
57 y.o. |
Buccal maxillary anterior gingival papilla |
Plexiform |
Biopsy |
Excision |
None after 1 year |
Borges, et al. [48] |
Male |
12 y.o. |
Labial mucosa |
Localized |
Biopsy
S-100 positivity |
Excision |
None after 1 year |
Kodiya, et al. [49] |
Male |
40 y.o. |
Soft palate |
Localized |
Biopsy |
Excision |
None after 2 weeks |
Pawar, et al. [50] |
Female |
25 y.o. |
Buccal maxillary posterior gingiva |
Localized |
Biopsy |
Excision |
None after 1 year |
Mahalle, et al. [51] |
Female |
37 y.o. |
Labial mucosa |
Plexiform |
Biopsy |
Excision |
None after 15 months |
Mahmud, et al. [52] |
Female |
73 y.o. |
Tongue |
Localized |
Biopsy
S-100 positivity |
Excision |
None after 1 year |
Sekhar, et al. [53] |
Female |
55 y.o. |
Hard palate |
Localized |
Biopsy
S-100 positivity |
Excision |
None after 1 year |
NS= not specified
When compared to former reported cases of solitary gingival neurofibromas, our case is peculiar in its clinical expression. Whereas most solitary gingival neurofibromas have been described as painless, localized, sessile or pedunculated exophytic gingival enlargements, our patient presented a painful and diffuse gingival lesion, mimicking periodontal pockets around the mandibular molars. Such clinical signs indicate the need for a well-conducted clinical evaluation, in order to establish proper differential diagnosis. In this particular case, the atypical feature may explain the initially delayed diagnosis.
Treatment of solitary neurofibromas consists in surgical excision, in order to solve a functional or esthetic disorder, or to avoid chronic traumatism, considering that a malignant transformation may occur. It is recommended to perform total tumor resection including 1 cm of surgical margins in order to reduce the risk of recurrence [19,21,28,37]. This approach seems to be the treatment of choice for small and accessible tumors as for this clinical case. To our knowledge, our case is the only one reported with a 7-year postoperative follow-up. Generally, sporadic neurofibromas are histologically identical to those encountered in NF1.
In conclusion, the present case report emphasizes the fact that neurofibromas may occur as sporadic lesions, without any NF1 characteristics and no family history. Their location and clinical features can be misleading, thus highlighting the need for histological examination as the gold standard to reach a definite diagnosis.
These neurofibromas may be the first manifestation of Neurofibromatosis type I; to our knowledge, at least one case has been reported in which the diagnosis of an oral neurofibroma led to the diagnosis of NF1. This points out the importance of a careful diagnostic approach when dealing with such lesions.
Considering the possible local complications and malignant transformation, it is crucial that dental clinicians and oral pathologists, conduct long-term follow-ups after surgical excision of such lesions, especially in NF1 patients [54] (Supplementary File).
Ethics approval and consent to participate: not applicable
Written informed consent was obtained from the participants.
Not applicable.
The authors declare that they have no competing interests.
Not applicable
- Concept and design: SMD, LB
- All authors performed the acquisition, analysis, and interpretation of data
- Drafting of the manuscript: SMD, LB
- All authors read and approved the final manuscript
Not applicable.
Not applicable.
The authors declare having no conflict of interests.
- Cunha KSG, Barboza EP, Dias EP, Oliveira FM (2004) Neurofibromatosis type I with periodontal manifestation. A case report and literature review. Br Dent J 196(8): 457-460.
- Gerber PA, Antal AS, Neumann NJ, Homey B, Matuschek C, et al. (2009) Neurofibromatosis. Eur J Med Res 14: 102-105. [Crossref]
- Go JH (2002) Benign peripheral nerve sheath tumor of the tongue. Yonsei Med J 43: 678-680.
- Gutmann DH, Aylsworth A, Carey JC, Korf B, Marks J, et al. (1997) The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA 278: 51-57.
- Jordan RC1, Regezi JA (2003) Oral spindle cell neoplasms: a review of 307 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 95: 717-724. [Crossref]
- Marocchio LS, Oliveira DT, Pereira MC, Soares CT, Fleury RN (2007) Sporadic and multiple neurofibromas in the head and neck region: a retrospective study of 33 years. Clin Oral Investig 11: 165-169.
- McClatchey AI (2007) Neurofibromatosis. Annu Rev Pathol 2: 191-216. [Crossref]
- Yohay K (2006) Neurofibromatosis types 1 and 2. Neurologist 12: 86-93. [Crossref]
- Ellis GL, Abrams AM, Melrose RJ (1977) Intraosseous benign neural sheath neoplasms of the jaws. Report of seven new cases and review of the literature. Oral Surg Oral Med Oral Pathol 44: 731-743.
- Pilavaki M, Chourmouzi D, Kiziridou A, Zarampouzas T, Dervelengas A (2004) Imaging of peripheral nerve sheath tumors with pathologic correlation: pictorial review. Eur J Radiol 52: 229-239.
- Wright BA, Jackson D (1980) Neural tumors of the oral cavity. A review of the spectrum of benign and malignant oral tumors of the oral cavity and jaws. Oral Surg Oral Med Oral Pathol 49: 509-522. [Crossref]
- Chrysomali E, Papanicolaou SI, Dekker NP, Regezi JA (1997) Benign neural tumors of the oral cavity: a comparative immunohistochemical study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 84: 381-390.
- Salla JT, Johann ACBR, Garcia BG, Aguiar MCF, Mesquita RA (2009) Retrospective analysis of oral peripheral nerve sheath tumors in Brazilians. Braz Oral Res 23: 43-48.
- Mahajan A, Dixit J, Bhardwaj A (2010) Gingival enlargement in neurofibromatosis type 1: a case report and literature review. J Contemp Dent Pract 11: 057-63.
- Lin V, Daniel S, Forte V (2004) Is a plexiform neurofibroma pathognomonic of neurofibromatosis type I? Laryngoscope 114: 1410-1414.
- Erlandson RA, Woodruff JM (1982) Peripheral nerve sheath tumors: an electron microscopic study of 43 cases. Cancer 49: 273-287.
- Peltonen J, Jaakkola S, Virtanen I, Pelliniemi L (1987) Perineurial cells in culture. An immunocytochemical and electron microscopic study. Lab Investig J Tech Methods Pathol 57: 480-488.
- Furniss D, Swan MC, Morritt DG, Lim J, Khanna T, et al. (2008) A 10-year review of benign and malignant peripheral nerve sheath tumors in a single center: clinical and radiographic features can help to differentiate benign from malignant lesions. Plast Reconstr Surg 121: 529-533.
- Bekisz O, Darimont F, Rompen E (2000) Diffuse but unilateral gingival enlargement associated with von Recklinghausen neurofibromatosis: a case report. J Clin Periodontol 27: 361-365.
- Apostolidis C, Anterriotis D, Rapidis AD, Angelopoulos AP (2001) Solitary intraosseous neurofibroma of the inferior alveolar nerve: report of a case. J Oral Maxillofac Surg 59: 232-235.
- Depprich R, Singh DD, Reinecke P, Handschel J (2009) Solitary submucous neurofibroma of the mandible: review of the literature and report of a rare case. Head Face Med 5:24.
- Dickersin GR (1987) The electron microscopic spectrum of nerve sheath tumors. Ultrastruct Pathol 11: 103-146.
- Ferner RE, O'Doherty MJ (2002) Neurofibroma and schwannoma. Curr Opin Neurol 15: 679-684. [Crossref]
- Hirbe AC, Gutmann DH (2014) Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol 13: 834-43.
- Ferner RE (2007) Neurofibromatosis 1. Eur J Hum Genet 15: 131-138. [Crossref]
- Poyhonen M, Niemela S, Herva R (1997) Risk of malignancy and death in neurofibromatosis. Arch Pathol Lab Med 121: 139-143. [Crossref]
- Zöller ME, Rembeck B, Odén A, Samuelsson M, Angervall L (1997) Malignant and benign tumors in patients with neurofibromatosis type 1 in a defined Swedish population. Cancer 79: 2125-2131.
- Bongiorno MR, Pistone G, Aricņ M (2006) Manifestations of the tongue in Neurofibromatosis type 1. Oral Dis 12: 125-129. [Crossref]
- Allen CM, Miloro M (1997) Gingival lesion of recent onset in a patient with neurofibromatosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 84: 595-597. [Crossref]
- Vincent SD, Williams TP (1983) Mandibular abnormalities in neurofibromatosis. Case report and literature review. Oral Surg Oral Med Oral Pathol 55: 253-258.
- Richards D (1983) Neurofibroma of the oral cavity. Br J Oral Surg 21: 36-43. [Crossref]
- do Nascimento GJF, de Albuquerque Pires Rocha D, G alvão HC, de Lisboa Lopes Costa A, de Souza LB (2011) A 38-year review of oral schwannomas and neurofibromas in a Brazilian population: clinical, histopathological and immunohistochemical study. Clin Oral Investig 15: 329-335.
- Zachariades N, Mezitis M, Vairaktaris E, Triantafyllou D, Skoura-Kafoussia C, et al. (1987) Benign neurogenic tumors of the oral cavity. Int J Oral Maxillofac Surg 16: 70-76.
- Cherifi H, Fournier B, Berdal A, McAlpin B, Sitbon I-Y, et al. (2019) Oral Manifestations of Neurofibromatosis Type 1. J Cosmet Dermatol Sci Appl 09: 41-55.
- Anneroth G, Sigurdson A (1983) Hyperplastic lesions of the gingiva and alveolar mucosa. A study of 175 cases. Acta Odontol Scand 41: 75-86.
- SHKLAR G, MEYER I (1963) NEUROGENIC TUMORS OF THE MOUTH AND JAWS. Oral Surg Oral Med Oral Pathol 16: 1075-1093. [Crossref]
- GarcĆa de Marcos JA, Dean Ferrer A, Alamillos Granados F, Ruiz Masera JJ, GarcĆa de Marcos MJ, et al. (2007) Gingival neurofibroma in a neurofibromatosis type 1 patient. Med Oral Patol Oral Cirugia Bucal 12: E287-E291.
- Papadopoulos H, Zachariades N, Angelopoulos AP (1981) Neurofibroma of the mandible. Review of the literature and report of a case. Int J Oral Surg 10: 293-297. [Crossref]
- Alivuotila L, Hakokari J, Visnapuu V, Korpijaakko-Huuhka AM, Aaltonen O, et al. (2010) Speech characteristics in neurofibromatosis type 1. Am J Med Genet A 152A: 42-51.
- O'Driscoll PM (1965) The oral manifestations of multiple neurofibromatosis. Br J Oral Surg 3: 22-31. [Crossref]
- Shapiro SD, Abramovitch K, Van Dis ML, Skoczylas LJ, Langlais RP, et al. (1984) Neurofibromatosis: oral and radiographic manifestations. Oral Surg Oral Med Oral Pathol 58: 493-498.
- Bruce KW (1954) Solitary neurofibroma (neurilemmoma, schwannoma) of the oral cavity. Oral Surg Oral Med Oral Pathol 7: 1150-1159.
- Alatli C, Oner B, Unür M, Erseven G (1996) Solitary plexiform neurofibroma of the oral cavity A case report. Int J Oral Maxillofac Surg 25: 379-380. [Crossref]
- Sinha R, Paul R, Sen I, Sikdar B (2002) A solitary huge neurofibroma of the soft palate. J Laryngol Otol 116: 637-638. [Crossref]
- Marocchio LS, Pereira MC, Soares CT, Oliveira DT (2006) Oral plexiform neurofibroma not associated with neurofibromatosis type I: case report. J Oral Sci 48: 157-160. [Crossref]
- Ohno J, Iwahashi T, Ozasa R, Okamura K, Taniguchi K (2010) Solitary neurofibroma of the gingiva with prominent differentiation of Meissner bodies: a case report. Diagn Pathol 5: 61.
- Suramya S, Shashikumar P, Kumar GS (2013) Solitary plexiform neurofibroma of the gingiva: unique presentation in the oral cavity. J Clin Diagn Res 7: 2090-2092.
- Borges AH, Correia RDM, Borba AM, Guedes OA, Estrela CRDA, et al. (2013) Unusual solitary neurofibroma on the lower lip of a child. Contemp Clin Dent 4: 512-5114.
- Kodiya AM, Ngamdu YB, Sandabe MB, Isa A, Garandawa HI (2013) Solitary isolated neurofibroma of the soft palate. J Surg Case Rep 2013. [Crossref]
- Pawar B, Tejnani A, Sodhi NK, Pendyala G (2013) A silent tumor of the gingiva: An unusual case report and surgical management with 1 year follow-up. J Indian Soc Periodontol 17: 510-513. [Crossref]
- Mahalle A, Gs Reddy M, Mohit Kheur S3, Bagul N4, Ingle Y5 (2016) Solitary Non Syndromic Oral Plexiform Neurofibroma: a Case Report and Review of Literature. J Dent (Shiraz) 17: 293-296. [Crossref]
- Mahmud SA, Shah N, Chattaraj M, Gayen S (2016) Solitary Encapsulated Neurofibroma Not Associated with Neurofibromatosis-1 Affecting Tongue in a 73-Year-Old Female. Case Rep Dent 2016: 3630153.
- Sekhar P, Nandhini G, Kumar KR, Kumar AR (2019) Solitary neurofibroma of the palate mimicking mucocele: A rare case report. J Oral Maxillofac Pathol 23: 23-26.
- Epstein JB, Schubert MM, Hatcher DC (1983) Multiple neurofibromatosis. Report of a case. Oral Surg Oral Med Oral Pathol 56: 560-562.






