Introduction: Bacterial infections contribute to acute exacerbation of COPD (AECOPD) in 50% of cases; Pseudomonas aeruginosa (PsA) is a probable pathogenic organism (PPM) causing acute or chronic infection in severe COPD patients. We examined whether sputum production and isolation of PPM (particularly PsA) influenced the LOS.
Method: A retrospective survey of sputum culture results and LOS in all COPD admissions to an acute hospital from January 2009 to December 2009, identified from prospectively collected records as part of a specifically funded service improvement pilot project and confirmed by coding (ICD: J44). Sputum cultures were attempted in all patients and collected in all sputum producers during that period. Patients were divided into sputum producers (SP) and non-producers (NSP). SP were further divided into patients with positive culture of PPM and non-pathogenic organisms. Median LOS was calculated to be 5 days and all groups were compared against this.
Results: There were 332 COPD admissions; 203 were SP. 122 had bacterial isolates. There were 37 pseudomonas growers. LOS was 5 or more days in: 121 SP vs 47 non-producers (p=0.000046); 79 PPM cultures vs 42 without bacterial growth (p=0.067); PsA growers vs. all admissions, (p=0.000744), PsA vs. all sputum producers (p=0.0126), PsA vs. all PPM (p=0.041).
Conclusions: There is a gradient of risk of longer LOS – from non-sputum producers with lowest risk, through sputum producers to those with PsA isolation in sputum being at the highest risk of healthcare resource utilization. Risk stratification by sputum production, and bacterial cultures followed by targeted treatment are therefore likely to reduce LOS and reduce re-admissions.
Chronic Obstructive Pulmonary Disease (COPD) is a common condition and it is estimated that 3.7 million people in the UK suffer from the illness. It is the second most common reason for emergency admissions to hospital [1]. One in eight acute medical admissions is from COPD exacerbation [2] and that constitutes more than one million bed days per year. It is the 5th leading cause of death in the world [3] and the incidence is projected to continue to rise due to increased smoking in women and is likely to take over as the third leading cause of death by 2020 [4,5]. The National Institute of Clinical Excellence (NICE) considers that the total cost of caring for the patients with COPD on the National Health Service (NHS) is in the range of £500 million per year and most of the expenditure is incurred by the cost of care provided for the patients in hospital setting [6].
According to the report of National Chronic Obstructive Pulmonary Disease Audit 2008, median duration of stay in hospital has improved and is now 5 days. [7] Improvement in duration of stay in hospital has been slow despite major investments and further reduction in hospital stay and earlier safe discharge is needed to provide care for the increasing number of patients and increasing number of hospital admissions. To achieve this goal we need to identify other factors and causes that may be responsible so those could be improved upon.
It is known that bacteria and viruses can be isolated from the patients admitted with acute exacerbation of COPD and antibiotics are effective treatment. [8] Pseudomonas aeruginosa is one of the possible pathogenic bacteria that can lead to an infective exacerbation; it tends to affect the patients with poorer lung functions and can also cause chronic infection. [9,10] Sputum cultures are sometimes not taken routinely, and patients are started on empirical antibiotic treatment, and mostly used first line therapies do not provide cover for pseudomonas infection, and thus we decided to determine whether Pseudomonas infection was one of the causes for a prolonged admission to hospital in a patient with COPD.
Study was based on a retrospective anonymised survey of a service improvement programme data approved by the Trust Governance board in a District General Hospital in the UK. All admissions coded under World Health Organisation (WHO) International Classification of Diseases (ICD-10) coding of J44.0 (COPD with Lower respiratory Tract Infection), J44.1 (COPD acute exacerbation (unspecified)) and J44.9 (COPD), from 1st January 2009 to 31st December 2009 were included. Information was collected regarding their date of admission and date of discharge or death and thus length of stay was determined by these dates. As part of the service improvement programme, all acute admissions for a COPD exacerbation were routinely reviewed by the specialist respiratory team during their inpatient stay. It is routine practice in hospital to collect sputum specimens for culture in a sterile container after washing oral cavity with water and then the specimens are sent to the pathology laboratory within the hospital. Information on the results of these cultures was collected from the laboratory database. The patients were divided into groups of sputum producers and nonproducers.
Patients who had a sputum specimen collected and cultured during admission were identified and noted as sputum producing group. Sputum producers were further divided into sputum producers with bacterial growth on culture and sputum producers without bacterial growth. In the group with bacterial growth, results of growth of pathogenic organisms were assessed for possible pathogenic micro-organisms (PPMs) and non-pathogenic micro-organisms (NPMs) and all PPMs were recorded, among PPMs, patients with Pseudomonas aeruginosa (PsA) were then analysed as a separate group and compared against all other groups for duration of admission. Patients with Haemophilus Influenzae were also compared against all other admissions to provide a control group from within the group with PPMs to compare against PsA growers. Median duration of admission was 5 days which is in keeping with national median duration of admission for COPD as shown by the report of National Chronic Obstructive Pulmonary Disease Audit 2008 and that was taken as a reference for expected length of stay to compare against for all groups. Ethics Committee approval was not required as it was an audit and a routine practice was being assessed and patient information was kept anonymous.
Statistical analysis
Median length of stay for all admissions were found to be 5 days and the patients were divided among two groups with length of hospital stay less than 5 days and the rest. Probability of a patient to have length of stay 5 or more days was modelled using logistic regression. In these models independent variables were presence or absence of certain micro-organisms in the sputum cultures and the statistical significance were considered for p-values ≤ 0.05. All reported p-values are two-tailed and based on large sample normal approximation of the parameter estimates in the logistic regression models. Data analysis was performed using R for Windows (version 2.7.2) software package.
There were 332 admissions coded as J44.0, J44.1 or J44.9 according to the WHO ICD-10 from 1st January 2009 to 31st December 2009, mean age 73.43 years (SD+/- 11.2). There were 181 males, mean age 73.32 years (SD+/- 10.66) and 151 females, mean age 73.55 years (SD+/- 11.85). 203 were sputum producers and 122 had positive bacterial isolates from the sputum cultures. There were 11 NPM isolates while the remaining were PPMs. There were 37 positive cultures for Ps A and 27 for Haemophilus Influenzae, details about the PPMs are as shown in table 1. In Figure 1, we plot an estimated distribution of the length of hospital stay for all admissions. From the figure, we can observe that nearly 80% of the admissions are below 10 days, but due to a few observations with very long length of stay, the average length of stay for all admissions was 7.69 days with SD +/- 9.69, which is rather high. As a robust measure, we consider the median length of stay, which is 5 days (IQR =7.0). This stay of 5 days was taken as standard or bench mark and all groups were compared for their length of hospital stay against this standard. 121 sputum producers stayed in hospital for 5 or more days against 47 non-producers, p= 0.0000466 suggesting significantly higher probability of longer hospital stay for the sputum producers. 79 patients with bacterial isolates from their sputum culture had 5 or more days of admission in hospital vs 42 patients with no growth on cultures, p=0.0675. On comparing Ps A group of 37 patients for 5 or more day admission vs all admissions, p= 0.000744; vs all sputum producers; p= 0.0126; vs all patients with bacterial isolates, p=0.0412. All of these p-values suggest that Ps A group of patients have a significantly higher probability of longer hospital compared to all admissions, all sputum produces and all patients with bacterial isolates, respectively. Other main isolate was Haemophilus influenzae (27 patients) and when this group was compared with all admissions for length of stay of 5 or more days, we get p= 0.892 suggesting no significant difference in the length of stay for these groups. Thus, these findings show that patients admitted with COPD exacerbation to hospital tend to have a longer duration of stay if they produce sputum, have PPMs isolated from sputum culture, and especially if they have infection with Pseudomonas Aeruginosa. Further, we have also observed that for the Ps A group, the average length of stay is 11.73 with SD +/- 9.8 and median is 9 days with IQR of 13.
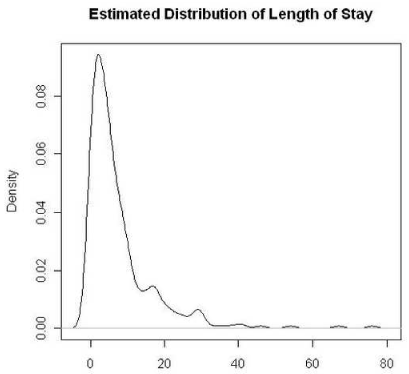
Figure 1. Estimated distribution of the length of hospital stay for all admissions.
Table 1. Details about the PPMs.
Probable Pathogenic Micro-organism (PPM) |
Number of isolates |
Pseudomonas aeruginosa |
37 |
Haemophilus influenzae |
27 |
Serratia |
10 |
Moraxella |
9 |
Staphylococcus auereus |
6 |
Escherichia coli |
6 |
Streptococcus pneumonia |
5 |
Klebsiella |
4 |
Methycillin resistant ataphylococcus auereus (MRSA) |
1 |
Proteus |
1 |
Mycobacteruim avium intracellulare |
1 |
COPD is a common condition and exacerbation of COPD is the second commonest cause of admission to hospital costing around £500 million per year to the NHS with more than one million bed days per year. A lot of investment has been made in this area with community matrons and COPD outreach teams to facilitate early but safe discharge of COPD patients from hospital. Despite all these efforts there has been only a reduction of one day in the median hospital stay with COPD admissions taking more than 5 times the number of bed days on comparison with Asthma [11].
Up to three quarters (74%) of patients attend admitted to the hospital make contact with their general practice in the month before admission and up to one third (33%) receive five or more courses of antibiotics in last twelve month before admission. [7] This provides an excellent opportunity to prioritise at risk patients for secondary care or more specialist opinion. It becomes even more important when we know that there has been an increase in the actual number of hospitalisations from COPD [2] and the number of re-admissions has increased as well [7].
It is known that pathogenic bacteria can be isolated from more than 50% of the patients admitted with acute COPD exacerbation (AECOPD) [12-14] and also that antibiotics are effective in treatment of AECOPD [15-17]. Haemophilus influenzae, Moraxella catarrhalis and Streptococcus pneumonia have been well established to cause infective exacerbations and can also cause intermittent or chronic infection in patients of COPD [18-21]. Gram negative organisms like Enterobacteriacae and Pseudomonas Aeruginosa (Ps A) are more commonly isolated from sputum in patients with more advance or severe COPD. Ps A can be isolated from sputum of up-to 15% of the patients with COPD [22-24]. It was suggested by Lode et al. [25] that presence of Ps A in sputum at the time of exacerbation is seen more commonly in patients with FEV1 of <35%, use of systemic steroids and recent antibiotic treatment, association of Ps A with poorer lung function was also observed by Timothy et al. [26]. Acquisition of new strain of Ps A has been shown to be associated with AECOPD, similar to the role described for H. influenzae, M. catarrhalis and strep. Pneumonia [27]. Timothy et al have also shown that patients with COPD can acquire different strains of Ps A and pattern of carriage can be different among different patients, and can be different among strains, some patients can acquire and clear the infection within a month other can do so over 2 years while some may develop chronic infection or colonisation with the mucoid strains. Acquisition of new strains of pseudomonas was also shown to be associated with an exacerbation [9]. Chronic effects of Ps A colonisation in COPD are not very well established, though it is considered to play an important role in advanced stable COPD [28], but in patients of Cystic Fibrosis it is known to contribute to the damage to the bronchial tree [29] and also to early mortality. It is an organism that can prove to be difficult to eradicate once infection is acquired [30,31], similarly colonisation or chronic infection in a setting of bronchiectasis has also been described.
We have observed in our study that 61.1% (203/332) of the patients admitted with COPD were sputum producers, and 60% of these sputum producers (122/203) had growth of PPMs in their sputum on culture, and that was associated with a longer stay in hospital, which would also mean that these patients are more unwell or their recovery is prolonged as compared with non sputum producers or those with no proven bacterial infection. Antibiotics have been shown to play an important role in the treatment of COPD exacerbations, but most of the treatments used as empirical therapy are focused to treating with antibiotics that are more potent against gram positive organisms like streptococci or gram negative organisms like H. influenzae and M. cattarhalis, which are believed to be more prevalent organisms causing infective exacerbation of COPD, but our observation in this audit was that Ps A was a common organism cultured from sputum of COPD patients, isolated from 37/332 i.e 11.1% of total COPD related admissions. It was even more prevalent among the patients with evidence of bacterial infection from sputum culture (either new acquisition or chronic) 37/122, 30.1% of all the positive sputum cultures.
Similar high Ps A isolation rate was observed by Timothy Murphy and colleagues [26] when they observed a group of COPD patients over 10 years, while Eller et al. [32], reported that Enterobacteriaceae and Pseudomonas was isolated in 48% of their COPD patients. Garcia-Vidal et al. [33] also reported in their study that 16.5% of total admission had Pseudomonas in sputum at the time of admission, but others have reported lower rate of isolation as well, like Sayiner et al. [34] had about 7% pseudomonas isolation in their COPD exacerbation related admissions. Lode et al [25] observed that Pseudomonas infection was associated with more severe COPD with FEV1 of less than 35%, use of systemic steroids and prior antibiotic treatment in the preceding 3 months while Garcia-Vidal et al. [33] did not see an association with previous antibiotic use but also found that such patients had higher BODE index (Body Mass Index, airflow obstruction, dyspnoea, exercise capacity), higher number of admissions in the last year and more were smokers. Pseudomonas is seen more commonly in patients with bronchiectasis and it is known that bronchiectasis can also be present along with severe COPD but Garcia Vidal et al. [33] in their study performed High Resolution Computed Tomography scan on randomly selected patients that constituted half of the patients studied, and no significant association with bronchiectasis and isolation of pseudomonas from patients of COPD was observed. We have not explored the probability of bronchiectasis in our population. Length of stay in hospital from COPD related admissions has improved with mean stay now about 7 days and median 5 days as mentioned in the National COPD Audit Survey Report [7], which is consistent with our findings, while Garcia-vidal [33] reported a much longer hospital stay of 11.0+/-8.7 days in their patients and also highlighted the repeated admissions in patients with PsA. Improvements have been made and there has been a reduction in the hospital stay of one day between the two national audits of 2003 and 2008 and other arrangements adopted in practice like hospital at home and early discharge teams have had an impact but there seems to be other factors along with increasing number of hospital admissions and re-admissions. We have described that length of stay is influenced by the production of sputum, infective nature of the exacerbation (based on positive sputum culture) and more so if there is Pseudomonas aeruginosa infection. It may be that these patients have a more severe disease and poorer lung functions, but at the same time antimicrobial agents mostly used as first line therapy mostly do not provide cover for PsA. It has been suggested in the European respiratory society task force recommendations on treatment of lower respiratory tract infections that patients with COPD exacerbations be screened for the factors that may make them more likely to have pseudomonas infection and then antimicrobial therapy be directed accordingly. This would lead to appropriately directed treatment early on in the admission and lead to a quicker recovery, early discharge and better outcome for these patients, and perhaps improve the readmissions also. But at the same time it has also been suggested that patients might clear the pseudomonas without antibiotic treatment or eradication therapy, and also the isolation of the Ps A is not always associated with exacerbation.
We believe that our data suggests that sputum producers with bacterial infections and especially pseudomonas is associated with longer admissions. In patients with severe COPD admitted to hospital admissions with an exacerbation, patients with repeated admissions or multiple courses of antibiotics, and also patients with previous growth of pseudomonas in their sputum, may need to be treated with antimicrobial agents that provide good cover against pseudomonas and that may have a beneficial effect on the outcome and shorten their duration of stay in the event of hospital. Subset of patients who become chronically colonised may play important role in stable disease and whether such patients benefit from anti pseudomanal therapy is not yet known. More well designed and larger studies are needed to look onto the effect of chronic colonisation with pseudomonas in patients with COPD and whether more should be done to eradicate the organisms when initially acquired. Despite the uncertainties, our data indicate that there is a gradient of risk of healthcare resource utilization (LOS) – from non-sputum producers with lowest risk, through sputum producers to those with bacterial isolates, those with PsA isolation in sputum being at the highest risk of healthcare resource utilization. They also suggest interventional studies of culture directed treatment of COPD exacerbations in primary care risk stratified by sputum production may be useful in addressing the burden of COPD exacerbation related healthcare resource utilization.
- Health Care Commission report, Clearing the Air 2006. http://www.cqc.org.uk/_db/_.../COPD_report1_200607272728.pdf
- Lost in Translation bridging the communication gap in COPD (June 2006) British Lung Foundation Survey. http://www.lunguk.org/.../British%20Lung%20Foundation/.../lost_in_translation2. pdf
- National Statistics (2006) Health Statistics Quarterly 30 http://www.statistics.gov.uk/downloads/theme_health/HSQ30.pdf
- Mannino DM1, Watt G, Hole D, Gillis C, Hart C, et al. (2006) The natural history of chronic obstructive pulmonary disease. Eur Respir J 27: 627-643. [Crossref]
- Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL et al. (2006) Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 27:188-207. [Crossref]
- Commission for Healthcare Audit and Inspection (2006) Clearing the Air: A national study of chronic obstructive pulmonary disease
- Report of The National Chronic Obstructive Pulmonary Disease Audit 2008: http://old.rcplondon.ac.uk/clinical-standards/ceeu/Current-work/ncrop/Pages/audit.aspx
- Wedzicha JA (2002) Exacerbations: etiology and pathophysiologic mechanisms. Chest 121: 136S-141S. [Crossref]
- Murphy TF, Brauer AL, Eschberger K, Lobbins P, Grove L, et al. (2008) Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 177: 853-860. [Crossref]
- Lung Report III (2003), British Lung Foundation.
- Murphy TF, Sethi S (1992) Bacterial infection in chronic obstructive pulmonary disease. Am Rev Respir Dis 146: 1067-1083. [Crossref]
- Seemungal T, Harper-Owen R, Bhowmik A, Moric I, Sanderson G, et al. (2001) Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 164: 1618-1623. [Crossref]
- Monsó E, Ruiz J, Rosell A, Manterola J, Fiz J, et al. (1995) Bacterial infection in chronic obstructive pulmonary disease. A study of stable and exacerbated outpatients using the protected specimen brush. Am J Respir Crit Care Med 152: 1316-1320. [Crossref]
- Saint S, Bent S, Vittinghoff E, Grady D (1995) Antibiotics in chronic obstructive pulmonary disease exacerbations. A meta-analysis. JAMA 273: 957-960. [Crossref]
- Chodosh S (1991) Treatment of acute exacerbations of chronic bronchitis: state of the art. Am J Med 91: 87S-92S. [Crossref]
- Lode H (1991) Respiratory tract infections: when is antibiotic therapy indicated? Clin Ther 13: 149-156. [Crossref]
- Sethi S, Evans N, Grant BJ, Murphy TF (2002) New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med 347: 465-471. [Crossref]
- Sethi S, Wrona C, Grant BJ, Murphy TF (2004) Strain-specific immune response to Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 169: 448-453. [Crossref]
- Murphy TF, Brauer AL, Grant BJ, Sethi S (2005) Moraxella catarrhalis in chronic obstructive pulmonary disease: burden of disease and immune response. Am J Respir Crit Care Med 172: 195-199. [Crossref]
- Fagon JY, Chastre J, Trouillet JL, Domart Y, Dombret MC (1990)Characterization of distal bronchial microflora during acute exacerbation of chronic bronchitis. Am Rev Respir Dis 142: 1004-1008. [Crossref]
- Anzueto A, Niederman MS, Tillotson GS, Group BS (1998) Etiology, susceptibility, and treatment of acute bacterial exacerbations of complicated chronic bronchitis in the primary care setting: ciprofloxacin 750 mg BID versus clarithromycin 500 mg BID. Clin Ther 20: 885-900. [Crossref]
- Martinez FJ, Grossman RF, Zadeikis N, Fisher AC, Walker K, et al. (2005) Patient stratification in the management ofacute bacterial exacerbation of chronic bronchitis: the role of levofloxacin 750 mg. Eur Respir J 25: 1001-1010. [Crossref]
- Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, et al. (2006) Infections and airway inflammation inchronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med 173: 1114-1121. [Crossref]
- Lode H, Allewelt M, Balk S, De Roux A, Mauch H, et al. (2007) A prediction model for bacterial etiology in acute exacerbations of COPD. Infection 35: 143-149. [Crossref]
- Murphy TF, Brauer AL, Eschberger K, Lobbins P, Grove L, et al. (2008) Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 177: 853-860. [Crossref]
- Sethi S, Evans N, Grant BJ, Murphy TF (2002) New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med 347: 465-471. [Crossref]
- Sethi S, Murphy TF (2008) Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 359: 2355-2365. [Crossref]
- Buret A, Cripps AW (1993) The immunoevasive activities of Pseudomonas aeruginosa. Relevance for cystic fibrosis. Am Rev Respir Dis 148: 793-805. [Crossref]
- Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL (2002) Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol 34: 91-100. [Crossref].
- Burns JL, Gibson RL, McNamara S, Yim D, Emerson J, et al. (2001) Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J Infect Dis 183: 444-452. [Crossref]
- Eller J, Ede A, Schaberg T, Niederman MS, Mauch H, et al. (1998) Infective Exacerbations of Chronic Bronchitis Relation between Bacteriologic Etiology and Lung Function. Chest 113: 1542-1548. [Crossref]
- Garcia-Vidal C, Almagro P, Romaní V, Rodríguez-Carballeira M, Cuchi E, et al. (2009) Pseudomonas aeruginosa in patients hospitalised for COPD exacerbation: a prospective study. Eur Respir J 34: 1072-1078. [Crossref]
- Eller J, Ede A, Schaberg T, Niederman MS, Mauch H, et al. (1999) Infective Exacerbations of COPD. Chest 115: 1481. [Crossref]
- Woodhead M, Blasi F, Ewig S, Huchon G, Ieven M, et al. (2005) Guidelines for the management of adult lower respiratory tract infections. Eur Respir J 26: 1138-1180. [Crossref]

