Granulomatosis with polyangiitis (GPA) is a potentially lethal Anti neutrophil cytoplasmic antibody (ANCA) associated with small-vessel vasculitis (AAV) involving the upper respiratory tract, lungs and renal vasculature. The presence of Renal cell carcinoma (RCC) as a manifestation of GPA is rare. RCC association with GPA has been reported in a few cases. Following the improved survival rates in patients with GPA, there is accumulating evidence to suggest an increased risk of different types of cancers. This is likely because of impaired immunosurveillance, direct oncogenicity of immunosuppressive agents, and perhaps malignant transformation of tissues undergoing chronic immune stimulation. Exposure to cyclophosphamide seems to be one of the main causes. Data suggests a standardized incidence ratio (SIR) of cancer in AAV of 1.6-2.0 compared to the general population. The most prominent cancers observed were urothelial cancer, leukemias, and non-melanoma skin cancer. This association might be related to chronic immunosuppression, however data showing the simultaneous occurrence of both suggests a common pathological pathways may be responsible. We report a patient with chronic GPA previously in remission, managed for a renal mass with histopathology showing clear cell RCC. Post-surgical development of active renal vasculitis questioned whether RCC was a trigger for GPA relapse. Awareness of cancer risk in AAV requires increased measures to prevent or screen for cancer and development of carcinogenic therapies.
renal cell carcinoma (RCC), ANCA associated vasculitis (AAV), granulomatosis polyangitis (GPA), immunosuppression
Granulomatosis with polyangiitis (GPA) is a rare, potentially lethal multisystem disease with an incidence of 3 per 100,00 people [1]. It belongs to the group of primary systemic ANCA-associated Vasculitides (AAV). Granulomatous inflammation and necrotizing vasculitis of small blood vessels lead to diverse clinical presentations with a classic triad of symptoms involving the upper and lower respiratory tracts as well as the kidneys. The disease can range from being mild to very severe and can be life threatening [2].
Severe disease is characterized by manifestations that threaten the function of vital organs, such as diffuse alveolar hemorrhage, glomerulonephritis, mononeuritis multiplex, sensorineural deafness, scleritis, or gangrene [3-6]. Non-severe disease, in contrast, is characterized by diseases affecting the sinuses, nares, and/or mucocutaneous surfaces, as well as pulmonary nodules, tracheobronchial disease, and arthritis. Despite this dichotomization, manifestations considered severe may be indolent and nonsevere manifestations may contribute significantly to disease morbidity. Sino-nasal, pulmonary, and musculoskeletal manifestations are the most common presenting symptoms. More often, patients with nonsevere disease develop severe disease after months to years of nonsevere manifestations [7]. This highlights the importance of early recognition and treatment initiation. The renal involvement is present 20 % at the time of diagnosis, however, occurs in 80 % eventually. Renal involvement is the most common severe manifestation and can lead to end-stage renal disease (ESRD), which is estimated to affect 30% of patients with renal involvement after five years [8]. The most common manifestation in the kidney is segmental necrotizing and crescentic glomerulonephritis, which often leads to rapidly progressive renal failure [9]. Older age, pulmonary, and kidney involvement are features associated with poor prognosis and increased mortality [10].
Generally, severe AAV is fatal if left untreated [11]. Even with treatment, mortality remains high. A recent meta-analysis [12,13] found that the standardized mortality ratio of death in AAV compared with the general population was 2.7 [95% confidence interval 2.3 to 3.2].
GPA was first described in the literature in a case report in the late nineteenth century and was previously known as Wegener’s granulomatosis [14]. The first case of GPA was reported by a German clinician, Heinz Klinger in 1931 who believed it to be a variant of polyarteritis nodosa, but it was Friedrich Wegener who proposed this as a separate pathology based upon the postmortem findings seen in three of his patients [15,16]. In GPA, ANCA is mainly directed against Proteinase 3 (PR3). There is strong evidence from various in vitro studies that ANCA plays a crucial role in the mediation of small vessel vasculitis [17]. With a standard therapy regimen, remission can be induced in about 70–90% of patients, but the typical course of the disease is with remission and exacerbations, which lead to the necessity for repeated courses of immunosuppressive treatment [18-21].
Cancer has also been linked to AAV as a potential causal or disease-triggering factor. Majority of data is pertained to GPA [7,22,23]. Its etiology is not well understood and likely involves multiple genetic and non-genetic determinants [24]. With the introduction of treatment regimens, combining high-dose glucocorticoids with cyclophosphamide or various other cytotoxic agents, disease remission for the majority of patients is achieved and the life expectancy of patients surviving the first year is approaching that of the general population [25]. Along with the better prognoses for AAV patients, potential long-term side effects of these therapies have progressively become a primary focus of interest. The de novo development of cancers associated with immunosuppressive therapy is a major concern for AAV. Previously published observations suggested that the overall incidence of cancer in treated AAV patients is 1.6-2.4 times higher than that of the general population [7,23-60]. Moreover, cancer type-specific analyses demonstrated an increased risk of bladder cancer [7,22,23] and leukemia [22,23], with those findings, spotlighting the known urothelial and hematological toxicities of cyclophosphamide [56]. Lymphoma [3,7,14,17,23,26] and non-melanoma skin cancer [22,23,26] incidences were also inferred. However, the reported risk levels varied, and those studies covered observation periods dating to the 1970s and 1980s that may no longer reflect the cancer risk of currently applied regimens.
The presence of a renal mass in patients with GPA is rare [27-31]; only a few cases have been reported. An analysis of 79 reported cases were done in 2000 with systemic vasculitis presenting as tumor-like lesion of which 28 patients had GPA with 5 having renal mass leading to nephrectomy. Pathological analysis of the masses revealed pseudotumor [32]. A retrospective statistical analysis performed in 1999 by Tatsis et al. on 477 patients with GPA showed malignant neoplasm in 23 of those patients [27]. In some cases, the disease developed simultaneously suggesting a temporal association with likely similar pathological pathway, while in other cases the RCC occurred several years after remission. This suggests that immunosuppression may be linked to this presentation; mostly noted with the use of cyclophosphamide.
We present a case of renal vasculitis that was temporally associated with the clinical presentation of renal cell carcinoma. Although such associations are rare, vasculitis affecting the kidney does appear to confer an increased risk of renal cancer, particularly in the presence of granulomatosis with polyangiitis.
64-year-old Caucasian male who underwent robotic assisted laparoscopic left radical nephrectomy for 8.7x6.7x8.1 cm mass arising from the lower pole of the left kidney with left renal vein extension, compatible with T3a Renal cell carcinoma in November 2020 (Figure 4), got admitted with fatigue, nausea, generalized weakness and poor appetite 3 weeks post-surgery. He complained of on and off arthralgia for years, which was treated with Acetaminophen with PRN Naproxen. His past medical history was significant for Wegener’s granulomatosis which was diagnosed in Sept 2004. He had sinusitis, dyspnea, and hemoptysis at that time. No tissue biopsy was in the records but as per the patient he had a chest x-ray followed by bronchoscopy and treatment was commenced based on alveolar hemorrhage and raised c-ANCA levels. He was never evaluated for renal involvement as per his Rheumatology records. His previous UA was negative for any proteinuria or RBCs, and his baseline creatinine was normal. He was managed with Cyclophosphamide and steroids from September 2004 for 1 year. Since 2007, he was started and maintained on oral methotrexate which was continued until 6 weeks back when it was withheld due to suspicion for renal cancer. He had hypertension since 2016 which was well controlled on carvedilol 12.5 mg twice a day. His social history was significant for alcohol abuse, but he was sober for the last 14 years and have never smoked. He was a salesperson, now retired. His presurgery creatinine which was done 1-week prior was 2.2 mg/dl, Urinalysis (UA) showed 3+ protein, 3+ blood, which was attributed due to his RCC.
His clinical examination showed a dry mucous membrane, with point of care ultrasound showing Inferior Vena Cava (IVC) of less than 1.5 cm and completely collapsing with a respiratory cycle, suggestive of hypovolemia. Initial blood work showed BUN of 108mg/dl, creatinine of 8.3 mg/dl, bicarbonate of 20 mmol/L, sodium of 133 mmol/L, potassium of 5 mmol/L, Chloride of 96 mmol/L, Phosphorus 8.4 mg/dl. UA showed a protein of 70 mg/dl, red blood cells were > 100/ high power field. Histopathology of the excised kidney showed clear cell renal carcinoma, WHO/ISUP nuclear grade 2 (Figure 4). Considering Acute Kidney Injury secondary to volume depletion, he was managed with Intravenous fluids.
With hydration, his clinical status improved as he started to eat and take fluids orally. His fatigue improved. Clinically looked euvolemic. In addition to this, his BUN and creatinine improved to 89 mg/dl and 7.3 mg/dl, respectively. Considering his AKI was most likely secondary to volume depletion with signs of improvement, he was discharged, however, ANCA levels were sent and pathology slide from his RCC specimen was requested for review for any possible glomerulopathy. Histopathology of the uninvolved renal parenchyma examined by Periodic Acid Schiff (PAS), silver and trichome stains showed rare glomeruli with segmental fibroid tuft necrosis and cellular crescent (Figure 1,2,3). There was patchy chronic interstitial inflammation and tubular injury. Later ANCA levels came out to be 1:640 positive for PR3 (level was 1115). He was managed with IV Methylprednisolone 500 mg for 3 doses followed by Prednisone 60 mg with a plan to taper over the next 6 months and Rituximab 375 mg/m2 weekly total of 4 doses. Over 4 weeks follow-up with completion of Rituximab, his creatinine declined to 2.5mg/dl, UA showed 6-10 RBC with UPCR of 0.5.
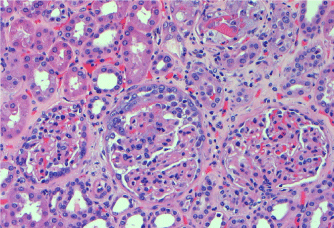
Figure 1: The glomerulus in the middle shows’ cellular crescent formation, H & E, 100 X
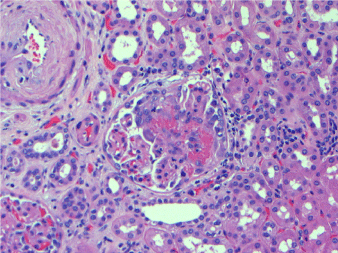
Figure 2. Glomerulus with segmental fibrinoid necrosis in the tuft. H&E, 200X
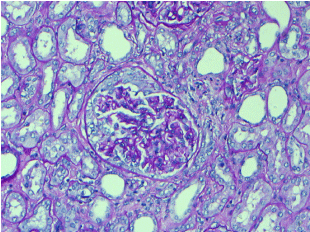
Figure 3. Glomerulus with cellular crescent. No hypercellularity or mesangial expansion was present in the tuft. PAS, 200X
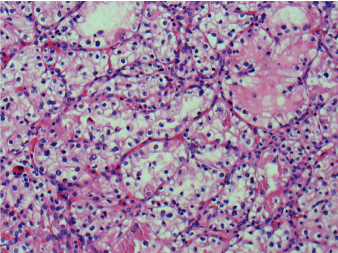
Figure 4. Renal cell carcinoma, clear cell type. H&E, 100X
Granulomatosis with polyangiitis, previously known as Wegener’s granulomatosis, is a systemic disorder characterized by necrotizing vasculitis of the respiratory tract and in most cases, the kidneys. The most common renal manifestation is segmental necrotizing and crescentic glomerulonephritis.
Malignancy is found in 5% of patients with vasculitis, the strongest association was seen with hairy cell leukemia, while colon, lung, and RCC seems to be the commonest solid tumor association [33]. The reason for this association is unclear, although mechanisms including immune complex formation with tumor antigens, shared tumor and vessel wall antigens, and impaired clearance of normally produced immune complexes have been postulated [34]. A 2019 review elucidated the link between autoimmune rheumatological diseases and cancer [35]. Reporting standardized incidence ratios (SIRs) for cancer in ANCA-associated vasculitis range from 1.6 to 2 in most studies [36], with most studies focusing on granulomatosis with polyangiitis (GPA). In studies that included the two phenotypes, that is, microscopic polyangiitis as well; however, GPA appears to have a higher risk of cancer [36].
According to Fain et al., [37], the malignancy rate in patients with any small vessel vasculitis (microscopic polyangiitis or GPA) was significantly higher than that of a normal age-matched general population (relative risk [RR] 7.5) and were more frequently associated with hematologic malignancies than solid tumors.
The biggest Asian study from Korea in 2019 searched the Korea National Health Insurance Claims Database to obtain data for 2097 AAV patients and evaluated the risk of cancer in AAV. The observations from this study showed a substantial portion of AAV patients (5.4%) developed cancer during follow-up period. The overall risk of cancer was significantly higher in patients with AAV (SIR 1.90) compared to the general population. For site-specific cancers, the risks of lung (SIR 2.23) and hematological (SIR 11.39) cancers were higher in AAV patients. Among AAV subtypes, patients with GPA had the highest risk of cancer. This increased risk of overall cancers was seen with cyclophosphamide, azathioprine/mizoribine, and methotrexate ever users [38].
Previous studies have reported that the administration of immunosuppressive agents is associated with the incidence of cancer [39,40]. Data from the Korean analysis [38] demonstrated that all treatments except rituximab were associated with an increased risk of overall cancer. This finding is in line with that of a recent publication by Daalen et al. [41] in which, rituximab treatment for AAV was not associated with the risk of cancer compared to the general population.
On the one hand, an increased risk of malignancy is well documented in patients treated with cyclophosphamide. A 33-fold increased risk for development of urinary bladder carcinoma was reported, however the risk is cumulative, dose dependent and related to duration of therapy. In GPA, the risk of both bladder cancer and leukemia increases with a cumulative dose of cyclophosphamide [22,23,42]. Patients who received cumulative doses greater than 36 g were at the highest risk for getting bladder cancer and acute myeloid leukemia [22]. On the other hand, recent studies suggested otherwise.
Faurschou and colleagues [43] reported the results of a registry-based study on long-term cancer risk following conventional immunosuppressive therapy in GPA. The authors had previously shown a greater-than-expected occurrence of Non-melanoma skin cancer (NMSC) among CYC-exposed patients and an increased incidence of bladder cancer and myeloid leukemia among patients treated with high cumulative CYC doses after a median follow-up of 6 years [22].
In this study [22], however, they extended the follow-up of their cohort to assess the risk of late-occurring malignancies. Among 293 patients, 255 had received CYC. The median follow-up was 9.7 years. The authors made important observations: first, the overall cancer risk did not increase in patients exposed to cumulative CYC doses of < 36 g, with the only type of malignancy occurring in excess being NMSC. Second, among those treated with a cumulative dose of >36 g, the overall cancer incidence increased, mainly due to an increased incidence of NMSC, bladder carcinoma, and myeloid leukemia. Third, in this (high-dose) group, the risk of NMSC remained high during the entire follow-up period (with cases still occurring >20 years after diagnosis), while that of myeloid leukemia and bladder cancer, respectively, peaked during the 5- to 9-year and >10-year latency periods. A dramatic risk of bladder carcinoma was observed >10 years after diagnosis in patients exposed to >36 g, with SIR of 29.0 (95% CI 10, 63). The authors could not assess the risk attributable to immunosuppressants other than CYC. Moreover, the cut-off of 36 g, chosen arbitrarily, cannot be used in clinical practice as a threshold for identifying the excess risk of cancer. Nevertheless, the results of this study are informative and underline the need for prolonged monitoring of cancer in GPA patients. However, they also show that judicious use of CYC, for instance by limiting it to the remission induction period, may carry a low cancer risk.
Furthermore, the recent 2019 Korean analysis [38], also showed that the risk of bladder cancer was not as elevated compared to previous studies. This nonsignificant association seems to be related to the lower cumulative dose of cyclophosphamide used, as cyclophosphamide is now usually used to induce remission in GPA. Accordingly, the cyclophosphamide dose used may not be sufficiently high to induce carcinogenesis in the bladder [44]. However, because patients treated with immunosuppressive agents generally have a higher risk of cancer and a distinct pattern of site-specific cancer incidence, it is essential to find measures to optimize the control of the disease while minimizing the use of immunosuppressants.
The introduction of glucocorticoid and CYC therapy in the 1960s dramatically improved the prognosis of affected patients, with 78% surviving at 5 years compared with a 1-year mortality in ∼80% of untreated patients [7]. No conventional immunosuppressants have emerged as valid alternatives to CYC for the induction treatment of AAV, except for MTX in early systemic disease. The introduction of biologics such as rituximab has offered an exciting perspective for AAV treatment; however, trials comparing rituximab and CYC as initial therapy for AAV demonstrated that the former is not superior in inducing remission and that its short-term toxicity is also not negligible [45]. Thus, CYC still remains a cornerstone and mainstay of treatment for GPA, markedly improving survival and remission rates [46]. Although the risks for urothelial cancer and leukemia were reported with cyclophosphamide, Renal Cell Carcinoma (RCC) has been reported in few cases and a direct connection is yet to be determined [28,29]. In 1996, Odeh reported for the first time the development of RCC after treatment with cyclophosphamide in a GPA patient [29]. In 2009, Deger et al. (28) reported RCC after eight years of GPA treatment with cyclophosphamide and azathioprine, proposing the possible connection between immunosuppressive therapy and renal cancer. Bumbasirevic et al. in 2011 reported a case of RCC with GPA after 13 years of remission [9], suggesting the same.
Several case reports have described this association and epidemiological work seems to confirm it [47]. Based on several case reports, malignancies have been suggested to trigger the onset of AAV [37,48-53], possibly accounting for the etiology in a small subset of patients [36]. The association between a preceding malignancy and the onset of AAV has also been investigated in larger studies, including retrospective case-control studies, which reported a 4.8%-10% prevalence of prior malignancies in patients with AAV [27,54,55].
One such study suggested that malignancy should be included in the differential diagnosis of patients presenting with renal vasculitis [54]. One possible explanation for the putative association between malignancy and AAV is that these conditions have common pathogenic pathways. Two studies have hypothesized on the common pathways in AAV and malignancies [27,46].
The finding of proteinase 3 within a human renal cell cancer line led to the analysis by Tatsis et al. [27] in 1999; reporting a significant association between RCC and GPA and suggested malignancy is in some cases a trigger for the development of WG, e.g., cytokine production by malignant cells may participate in the etiology of autoimmunity and vasculitis. In this study, 477 patients with GPA were analyzed; the odds ratio for malignant neoplasm in this group was 1.8, but the odds ratio for RCC was 8.7 when the comparison group was patients with Rheumatoid arthritis. The disease occurred simultaneously in five out of seven patients with GPA. 91% of the cases with GPA and malignancy were PR3 c-ANCA positive, although PR3 was not found in the malignant tissues of eight of the GPA patients who were tested; however, they did find an 18-fold increase in the risk of simultaneous (i.e., within 3 months) occurrence of malignancy and GPA compared to the risk of simultaneous occurrence of malignancy and rheumatoid arthritis (RA). In separate analyses according to malignancy sites, an increased risk of renal cell carcinoma was observed in their study.
Faurschou et al. hypothesized that the increased prevalence of NMSC preceding GPA could be due to an imbalance in immune status; they stated that it would be tempting to speculate that a state of acquired immunological dysfunction predisposes to both conditions, given the well-established association between immunosuppression and development of NMSC [55]. This immune imbalance may also contribute to the higher incidence of malignancies after a diagnosis of AAV, in addition to the effects of some immunosuppressive agents [36]. However, because AAV is generally treated with immunosuppressive therapy, investigating the relationship between AAV and malignancy becomes complicated once the patient has been diagnosed with AAV. Previous studies have yielded inconsistent results regarding the putative association between malignancy and subsequent AAV. These controversies should be resolved before researchers can investigate the relationship between these 2 conditions in further detail.
Our patient had GPA diagnosed 16 years back treated for a year with cyclophosphamide followed by chronic immunosuppression with methotrexate, now developing RCC, suggesting that chronic immunosuppression might be responsible for that, although we do not know what the cumulative dose of CYC was given to him. The literature search so far has shown such associations with the GPA itself in remission. The fact that our patient simultaneously had RCC and active renal vasculitis which responded well to rituximab raises the possibility of RCC being a trigger for the relapse of GPA (Table 1).
Table 1. Malignancy risk in antineutrophil cytoplasmic antibody-associated vasculitis in previous studies




