Despite progressive improvements in pregnancy-related health care in high-income countries and the concomitant decline in maternal mortality, heart failure (HF) remains a principal cause of non-obstetric maternal deaths in women with a pre-existing or undiagnosed cardiac disease. Although a structurally and functionally normal heart tolerates pregnancy-related physiological stress, the presence of cardiac diseases can deteriorate cardiac function leading to HF (the inability of the heart to function as a pump). With increasing ageing obstetric population, obesity, immigration and survival to adulthood of babies operated for congenital heart disease, the need to identify women at risk of developing heart failure in pregnancy (HFP) and to plan their careful management will also inevitable increase. Thus, in pursuit of safe motherhood, there is a need for a better understanding of aetiology, risk factors, pathophysiology, diagnosis and management of HFP. In the present paper, we review published evidence on HFP to advance the understanding of its clinical status as well as to highlight areas of limited knowledge that could benefit from additional research.
Key words
heart failure in pregnancy
Abbreviations
ACE-I: Angiotensin Converting Enzyme – Inhibitors; AHA : American Heart Association; ARBs: Angiotensin Receptor Blockers; BACH: Boston Adult Congenital Heart; BP: Blood Pressure; bpm: Beats per minute; CARPREG: Cardiac Disease in Pregnancy; CEMACH: Confidential Enquiries into Maternal Deaths; CHD: Congenital Heart Disease; CO: Cardiac Output; CT: Computed Tomography; CV: Cardiovascular; CVD: Cardiovascular Diseases; DCM: Dilated Cardiomyopathy; ESC: European Society of Cardiology; HF: Heart Failure; HFP: Heart Failure in Pregnancy; LA: Left Atrium; LV: Left Ventricular; MRI: Magnetic Resonace Imaging; NYHA: New York Heart Association; PH: Pulmonary Hypertension; PO: Pulmonary Oedema; PPCM: Peripartum Cardiomyopathy; RV: Right Ventricular; SVR: Systemic Vascular Resistance; WHO: World Health Organization; ZAHARA: Zwangerschap bij Aangeboren HARt Afwijkingen (Pregnancy in women with CHD II risk index)
Non-obstetric causes of maternal deaths account for a significant proportion of pregnancy-related mortality in both developed and developing countries [1,2]. Globally, they account for ~25% of deaths in Europe and the United States with considerably higher rates in Southern Asia (~29.3%) and Sub-Saharan Africa (~28.6%) [3]. In particular, previously known or undiagnosed cardiac diseases complicate about 1 to 4% of pregnancies [4]. Physiological stress during pregnancy and the peripartum period may precipitate or exacerbate pre-existing cardiac diseases leading to deterioration in ventricular myocardial function and depressed cardiac output that is insufficient to satisfy the body’s metabolic demands [1]. Thus, a working knowledge about normal morphological and functional changes in pregnancy is critical for timeous recognition of cardiac diseases in the management of affected pregnant women [4]. Moreover, familiarity with treatment of commonly encountered cardiac diseases during and after pregnancy, and contraindicated HF medication is becoming increasingly important in the pursuit of safe motherhood. This review therefore appraises published evidence on the aetiology, risk factors, aetiology, pathophysiology, diagnosis and clinical management of HFP.
Definition
The American Heart Association (AHA) [5] defines HF as a complex clinical syndrome resulting from any structural and/or functional impairment of ventricular filling or ejection of blood. The European Society of Cardiology (ESC) further characterizes HF as having typical symptoms of breathlessness, ankle swelling and fatigue that may be accompanied by signs of elevated jugular pressure, pulmonary crackles, and peripheral oedema, which leads to reduced cardiac output (CO) [6]. Although HF may result from a diversity of pathologies including disorders of the pericardium, myocardium, endocardium, heart valves or great vessels or from certain metabolic or congenital abnormalities, many patients with HF usually exhibit symptoms due to impaired left ventricular (LV) myocardial function [5,6]. During pregnancy, anatomic and hemodynamic perturbations impose a physiological stress on the cardiovascular system, which in some women, may exacerbate pre-existing cardiovascular conditions such as hypertension or precipitate the development of new cardiovascular conditions such as cardiomyopathy, which if unattended may progress into HFP [7]. Thus, in this context, HFP may be defined as a new onset of HF during pregnancy and up to six months postpartum in the absence of any other known cause.
Accurate epidemiological data on maternal deaths are available in a few countries that have pursued statutory notification of all maternal deaths [8]. In the United Kingdom (UK), the Confidential Enquiries into Maternal Deaths (CEMACH) reported an increase in the overall rate of mortality due to cardiac causes from 7.3 per million births in the 1982-1984 triennium [9] to 22.7 per million births in the 2003-2005 triennium [10]. A greater majority of this increase is attributed to acquired heart diseases, which accounted for 4.7 per million births to 20.8 per million births within the two trienniums respectively [9,10]. Maternal mortality rates per 100,000 live births in the U.K. are in single digits [11], By contrast, South African data, also accumulated based on statutory and confidential enquiry, reports maternal mortality rates of 179 per 100,000 live births. While the HIV related mortality (40% of all deaths) dominate the South African data, medical and surgical disorders account for 8.8%, which is the fourth leading cause of all deaths after HIV, haemorrhagic and hypertensive deaths [12]. Cardiac deaths account for 36.5% of deaths due to medical and surgical disorders [11].
In the U.S., post hoc analysis of the Nationwide Inpatient Sample (NIS) 2001-2011 data on 50 million pregnancy-related hospitalization reports the overall incidence rate of HFP of 112 per 100,000 pregnancy-related hospitalizations. Of these, ~60% of HFP occurred postpartum, 27.3% during delivery and 13.2% during antepartum [13]. Genetic and social variations also influence incidence of HFP. In the US, significant disparities have been identified in the incidences of HFP secondary to peripartum cardiomyopathy (PPCM): African Americans (1: 1,421); Asians (1: 2,675); Caucasians (1: 4,075); and Hispanic origin (1: 9,861) [14]. In terms of the underlying disease, hypertensive heart disease is the most prevalent complicating ~2 to 8% of all pregnancies in the western world, predominantly in Latin America and the Caribbean, in which the disease accounts for 25% of all maternal deaths [15]. By contrast, in developing countries, rheumatic heart disease is predominant but rare in the Western world [16].
Risk factors
Women with pre-existing cardiac diseases are at an increased risk of developing HFP. In these women, it is recommended to have a preconception discussion on contraception and on the impact of the disease on pregnancy [8]. Women with New York Heart Association (NYHA) Functional Class III or IV have a mortality rate ≥ 7% and morbidity rate >30% during pregnancy, and thus, pregnancy is usually not advisable in these women [4]. The most common cardiac complication frequently detected in pregnant women include arrhythmias, thromboembolic events and HF [17]. Early clinical trials investigating the risk of cardiac complications in pregnancy identified NYHA functional class and cyanosis as important risk factors [18-20]. At present, several pregnancy risk indices assessing the likelihood of developing cardiac complications have been developed based on large cohorts of pregnant women [21-27].
Pregnancy risk indices
Cardiac Disease in Pregnancy (CARPREG) investigators [21] developed the initial prospective 4-item pregnancy risk index based on clinical outcomes in women diagnosed with congenital and/or acquired heart diseases: prior cardiac events, NYHA functional class > II or cyanosis, left heart obstruction and systemic/sub-aortic ventricular systolic dysfunction (Table 1). The Boston Adult Congenital Heart (BACH) group investigated outcomes of women with congenital heart disease, an in addition to the CARPREG risk factors, identified smoking history and decreased sub-pulmonary ventricular function and/or severe pulmonary regurgitation [22]. ZAHARA (Pregnancy in women with CHD II risk index) investigators [23] developed an expanded 8-item weighted pregnancy risk score (Table 2).
Table 1. CARPREG pregnancy risk index for cardiac complications (HF: Heart Failure; NYHA: New York Heart Association)
Risk factor (1 point each) |
Total points |
Risk for cardiac complications (%) |
Prior event (arrhythmia/stroke/HF) |
0 |
5 |
NYHA Class > II or cyanosis |
1 |
27 |
Left heart obstruction |
> 1 |
75 |
Systemic ventricular dysfunction |
|
|
Table 2. ZAHARA pregnancy risk index for cardiac complications (AV: Arteriovenous; NYHA: New York Heart Association)
Risk factor |
Weighted points |
Total points |
Risk of cardiac complication |
Prior arrhythmias |
1.50 |
0 |
2.9 |
NYHA Class > 2 |
0.75 |
0.5-1.5 |
7.5 |
Left heart obstruction |
2.50 |
1.51-2.50 |
17.5 |
Cardiac medication at baseline |
1.50 |
2.51-3.50 |
43.1 |
Systemic AV valve regurgitation |
0.75 |
> 3.51 |
70.0 |
Sub-pulmonary AV valve regurgitation |
0.75 |
|
|
Mechanical valve prosthesis |
4.50 |
|
|
Cyanotic heart disease |
1.00 |
|
|
Recently, the updated ESC guidelines on management of cardiovascular diseases (CVD) during pregnancy created lesion-specific risk factors based on modified World Health Organization (WHO) classification of cardiac lesions [28] (Table 3), which is now a widely used tool for pregnancy risk prediction in several studies [29]. The WHO classifies cardiac lesion into four: low (I), medium (II), high (III) and lesion contraindicated in pregnancy (IV) [30].
Table 3. The ESC (Modified WHO) pregnancy risk prediction model (LV: Left Ventricular; LVEF: Left Ventricular Ejection Fraction; PAH: Pulmonary Arterial Hypertension; RV: Right Ventricular; NYHA: New York Heart Association: Adapted from the ESC guidelines on the management of CVD in pregnancy [28])
WHO class |
Details of risk factor |
I |
Uncomplicated/mild pulmonary stenosis; patent ductus arteriosus; mitral valve prolapse; successful repaired simple lesions. |
II (if well and uncomplicated) |
Unrepaired atrial/ventricular septal defect; unrepaired tetralogy of Fallot |
II-III (depends on individual) |
Mild LV impairment; native/tissue valvular heart disease (not considered in class I and IV); Marfan syndrome without aortic dilation; aorta < 45 mm with bicuspid aortic valve disease. |
III |
Mechanical valve; systemic RV; Fontan circulation; unrepaired cyanosis; other complex congenital heart disease; aortic dilation 40-45 mm in Marfan syndrome; aortic dilation 45-50 mm in bicuspid aortic valve disease |
IV (Pregnancy Contraindicated) |
PAH from any cause; severe systemic ventricular dysfunction (LVEF<30%, NYHA III-IV); severe mitral stenosis; severe symptomatic aortic stenosis; Marfan syndrome + aorta dilated > 45 mm; aorta dilation > 50 mm in aortic disease associated with bicuspid aortic valve; native severe coarctation of the aorta |
Despite remarkable improvement in the understanding of pregnancy risk factors in women with undiagnosed or known HF including the development of risk indices, clinical judgment remains a critical aspect of risk stratification. In practice, other pregnancy variables either unknown or not well described in literature whose effect on maternofoetal outcomes have not been captured in current pregnancy risk indices may require assessment by obstetricians and cardiologists with experience in pregnancy [17].
Obstetric and perinatal risks
In addition to pregnancy risk factors, obstetric and perinatal outcome have risks that may also precipitate or exacerbate HF and associated adverse outcomes in pregnancy. About 32% of women with congenital heart diseases are at risk of adverse obstetric complications including pre-term delivery, premature rapture of membranes and postpartum haemorrhage [29]. Cyanotic heart disease in pregnancy has been associated with miscarriages and low-birth rates in 43% of all pregnancies, and haemorrhaging during delivery [31], while coarctation of the aorta has been associated with increased risk for hypertension, preeclampsia, and HF [32]. On the other hand, risk factors for perinatal complications include poor maternal NYHA functional class, left heart obstruction, maternal age < 20 or > 35 years, multiple gestations, smoking during pregnancy and anticoagulant therapy [26]. Perinatal risks may be further increased by the presence of obstetric risks such as history of premature delivery, rapture of membranes, caesarean delivery, antepartum haemorrhaging > 12 weeks gestation, and febrile illness or uterine/placental abnormalities [17,26].
Aetiology
Several cardiovascular pathologies that may HFP can be classified into two main categories: (a) pregnancy-specific aetiologies of HF – preeclampsia, peripartum cardiomyopathy and amniotic fluid embolism; and (b) non-pregnancy related aetiologies that may become co-morbid diseases and complicate pregnancy [17].
Pregnancy-specific causes
Preeclampsia: The South African statutory and confidential enquiry on maternal mortality reports that pulmonary oedema (PO) caused by preeclampsia together with cerebrovascular haemorrhage is the leading cause of hypertensive maternal deaths [12]. In pregnant women, preeclampsia may cause hyper-dynamic circulation and increase LV contractile function [33]. Raised systemic vascular resistance (SVR) may increase left atrium (LA) filling pressures, and in combination with intravenous fluid administration, increases the risk of developing PO in the setting of diastolic dysfunction [34]. The LV has poor toleration of intravenous fluid load leading to a rapid rise in left side filling pressure in the absence of similar observable changes in the right heart [33,35]. Although a mild impairment of the systolic function may be seen in severe preeclampsia, in most cases it is transient [17].
Peripartum cardiomyopathy: Peripartum cardiomyopathy (PPCM) is a leading cause of HFP during the last month of pregnancy and up to six months after pregnancy in women without known CVD characterized by depressed LV systolic function (LVEF < 45%) [36,37]. Typically, but not always, PPCM is a form of dilated cardiomyopathy (DCM) that develops in the absence of any other identifiable cause of HF [38]. Its risk factors include older maternal age, multifetal gestations, and hypertensive disorders [39], obese and multiparous women with preeclampsia aged > 30 years [36]. The mechanism through which PPCM leads to HF is partially understood but myocyte apoptosis, inflammatory changes inducing oxidative stress and development of auto-immunity to proteins of cardiac origin have been suggested as the causative mechanisms [40-42]. Association between PPCM, preeclampsia and multiparity linked with an imbalance in angiogenic and anti-angiogenic factors, suggest that this mechanism may be one of the pathways leading to the development of cardiomyopathy [43].
Amniotic fluid embolism: Amniotic fluid embolism is an uncommon obstetric labour complication where amniotic fluid and/or foetal squames enter the maternal bloodstream triggering an acute onset of a syndrome marked by cardiovascular collapse, PO, seizure activity, and bleeding diathesis [44]. Although the mechanisms leading to HF are poorly understood, the release of large amounts of amniotic fluid and foetal squames into the maternal circulation triggers acute pulmonary hypertension (PH) followed by LV failure that needs inotropic support for a protracted period. Arrhythmias are also common in amniotic fluid embolism [44].
Non-pregnancy related causes: Non-pregnancy related causes of HF might be classified in terms of the main causative mechanism: (a) diseases increasing vascular resistance; (b) aortic root pathologies; and (c) heart disease itself due to obstruction or ventricular failure/congenital cardiac abnormalities and proximal vasculature [17].
Diseases increasing vascular resistance: Diseases that lead to an increase in vascular resistance include hypertensive cardiomyopathy and pulmonary congestion and right heart failure. Hypertensive cardiomyopathy may cause diastolic dysfunction and together with increased pregnancy-related preload predisposes some women to mild PO during 32-34 week gestation when plasma volume peaks [45,46]. Chronic hypertension may also increase the risk of preeclampsia, placental abruption, restricted foetal growth and pre-term birth [47]. PH and right HF may initially present with signs of exertional dyspnoea, weakness and recurrent syncope followed by signs of right HF such as increased jugular venous pressure, loud second heart sound, hepatomegaly and peripheral oedema. Primary PH increases the risk of acute right HF after delivery and may precipitate sudden cardiac death [48].
Aortic pathologies
Aortic pathologies may be classified into medical disorders associated with the risk of dissection such as inflammatory diseases of the aorta such as aortic arch syndrome and atherosclerotic disease. Marfan syndrome and Takayasu’s arteritis are the two common aortic arch syndrome and atherosclerotic diseases presenting during pregnancy [49]. Arterial dissection and acute aortic regurgitation are common acute manifestation of Marfan’s syndrome during pregnancy especially in women with dilated aortic root [50].
Heart diseases
Heart diseases that can cause HFP include disorders causing ventricular failure, valvular lesions, and congenital abnormalities affecting the heart and proximal vasculature [17]. Cardiomyopathies and ischemic heart disease are common causes of LV failure during pregnancy, and in particular, cardiomyopathies are a principal cause of maternal deaths both during and after pregnancy [25,51]. Besides PPCM, which is a pregnancy-specific cause of HF, other cardiomyopathies such as hereditary, drug-induced, autoimmune and infective cardiomyopathies can lead to ventricular failure and HFP [52]. Hereditary cardiomyopathies – hypertrophic, dilated and RV arrhythmogenic cardiomyopathies represent genetic mutations in the cardiac sarcomere, myocyte and desmosomes respectively. Hypertrophic cardiomyopathy is well tolerated in pregnancy in the absence of related symptoms. It may manifest as increased LV wall diameter that is not accounted for by co-occurring hypertension or valvular disease [53,54]. Pregnancy complicated by DCM is predicted by the severity of pregnancy symptoms. RV arrhythmogenic cardiomyopathy increases the risk of sudden cardiac death, which is not altered by pregnancy [55].
Drug-induced cardiomyopathy may also lead to the development HFP. Drugs such as alcohol, cocaine, amphetamines, methamphetamine, catecholamine, ephedrine, zidovudine, chloroquine, cyclophosphamide and certain antimitotic drugs used during pregnancy are rarely regarded as a cause of acute HF but may potentially induce cardiomyopathy [17]. Ischemic heart disease on the other hand, is an uncommon complication in pregnancy. Usually, the disease presents with symptoms of ischemia rather than HF, and commonly, the major risk to the mother is death due to acute myocardial infarction [17].
Besides the loss of LV function, valvular lesions such as post rheumatic valvular heart disease may result in PO during pregnancy and ultimately HF. Hyperdynamic circulation and increased plasma volume during pregnancy, which peaks at 34 weeks gestation, may precipitate PO. Increased pulse rate further aggravated by labour pain leads to decreased LV filling times in turn increasing the risk of HF in women with stenotic mitral valve disease. In addition, arrhythmia and co-morbidities such as anaemia, hypertension and thyroid disease all increase the risk of PO in women with valve disease. Arrhythmias, obstetric haemorrhage are complications of valvular diseases [25].
Finally, uncorrected CHD rarely complicates pregnancy or lead to HF during pregnancy. However, uncorrected Marfan syndrome with aortic root dilation and Eisenmenger syndrome carry the greatest risk of death in pregnant women due to dissection or HF [56]. Congenital stenosed valves, cyanotic heart disease without PH, systemic RV or Fontan circulation carry a moderate risk of HF while septal defects and repaired coarctation carry the lowest risk of HF [57].
Physiology during pregnancy
Pregnancy induces major hemodynamic alterations necessary to meet progressively increasing maternofoetal metabolic demands. These hemodynamic alterations include increases in blood volume, stroke volume, and reduction in SVR and blood pressure. Table 4 provides a summary of major hemodynamic perturbations at different stages of pregnancy (normal pregnancy and during layout and delivery) and postpartum [4].
Table 4. Hemodynamic alterations during pregnancy and postpartum period (Adapted from Pushpalatha et al. [4])
| Hemodynamic variable
|
Change during normal pregnancy
|
Change during labour and delivery
|
Change during postpartum
|
Plasma volume |
↑ 50% to 50% |
↑ |
↓ Auto-diuresis |
Heart rate |
↑ 10 to 15 bpm |
↑ |
↓ |
Cardiac output |
↑ 30% to 50% > baseline |
↑ Additional 50% |
↓ |
Blood pressure |
↓ 10 mmHg |
↑ |
↓ |
Stroke volume |
↑ 1st-2nd ↓ 3rd Trimester |
↑ 300-500 mL per contraction |
↓ |
Systemic vascular resistance |
↓ |
↑ |
↓ |
Hemodynamic alterations in pregnancy begins in the fifth lasting to the eight week of gestation because of systemic vasodilation to reach its peak in the late second trimester. In women with pre-existing cardiac disease, cardiac decompensation coincides with this peak. Plasma volume reaches a maximum of 40-50% above baseline at 24 weeks gestation. The increase in plasma is greater than the increase in red blood cells leading to a decrease in haemoglobin concentration to create the perception of anaemia during pregnancy [4]. Cardiac output (CO) also rises to a maximum of 30 to 50% above baseline, peaks towards the end of the second trimester and then plateaus until delivery. Mechanisms leading to increased CO include increased plasma volume; decreased afterload due to reduced SVR; and increasing maternal heart rate by 10 to 15 bpm [58,59]. Stroke volume on the other hand increases in the first and second trimester before decreasing in the third trimester due to uterine compression of the inferior vena cava. By the end of the second trimester, blood pressure falls by ~10 mm Hg below baseline because of a reduction in SVR, and the addition of new uterine and placental blood vessels [8].
Uterine contractions, positioning (left lateral/supine), pain, anxiety, exertion, haemorrhaging, and uterine involution result in significant haemodynamic alterations during labour and the postpartum period. In addition, anaesthesia, analgesia, haemorrhage and infection increase cardiovascular stress. Each uterine contraction ejects 300 to 500 mL of blood into the general circulation. An increase in stroke volume leads to a rise on CO by 15% in early labour, 25% during stage 1, by 50% during expulsive efforts and up to 80% in the early post-partum because of auto-transfusion associated with uterine involution and resorption of leg oedema [60]. Systolic and diastolic blood pressure increases by 15-25% and 10-15% respectively during uterine contractions [28]. Maternal pain and anxiety contributes to an increase in mean arterial pressure. Hemodynamic stress also increases because of blood loss during delivery, about 300-400 mL for vaginal delivery and 500-800 mL for caesarean section [4]. During the postpartum period, relief of the compressed inferior vena cava causes an increase in venous return, in turn increasing CO causing brisk diuresis. The hemodynamic changes revert to pregnancy baseline within 2-4 weeks after vaginal delivery and 4-6 weeks after caesarean delivery [4].
Diagnosis
Clinical assessment: Signs and symptoms resulting from hemodynamic changes often complicate cardiac assessment of the pregnant patient. Common symptoms of pregnancy such as breathlessness and fatigue mimic those of HF. Clinical signs of mild dependent oedema, minimally raised jugular venous pressure, collapsing pulses and ejection systolic murmur are common in pregnancy as well as in HF [61,62]. However, some signs and symptoms that may be abnormal during pregnancy assist in cardiac assessment for HFP. These signs and symptoms include extreme breathlessness, significant oedema, fourth heart sound, diastolic murmurs, jugular venous pressure > 2 cm and persistent tachycardia > 100 bpm. Any one of these signs and symptoms should prompt further clinical evaluation for HFP [63].
Diagnostic tests: The ESC Guidelines on the management of CVD during pregnancy recommend the diagnosis of HFP should be based on the combination of careful assessment of the patient’s history, physical examination and cardiac imaging [28].
History and physical examination: Taking a careful personal and family history can help clinician to identify many cardiac disorders including cardiomyopathies, Marfan syndrome, congenital heart disease, juvenile sudden death or Brugada syndrome [5,6]. Patient history should provide specific information about possible sudden deaths in the family. The assessment of dyspnoea is important for diagnosis and prognosis of valvular lesions and HF. A thorough physical examination that considers hemodynamic changes taking place during pregnancy is mandatory for the diagnosis of HF. Physical examination should include auscultation for new murmurs, changes in murmurs and signs of HF. The presence of dyspnoea during pregnancy or new pathological murmur indicates the need for echocardiography assessment. Physical examination should also include measurement for the blood pressure as well as evaluation for proteinuria in patients with a family history of hypertension or preeclampsia. Finally, oximetry should be considered in patients with CHD [28].
Electrocardiography: Electrocardiography (ECG) is a very common and useful diagnostic tool throughout pregnancy for assessing women with complaints of chest pain or arrhythmias. Usually, a majority of pregnant women have normal ECG. During pregnancy, the heart is rotated towards the left and surface ECG shows 15-20 left axis deviation. Common ECG findings in pregnant women include transient ST segment and T wave changes, Q wave due to diaphragmatic elevation, inverted T waves in lead III, attenuated Q wave in lead AVF and inverted T waves in leads V1, V2 and occasionally V3. These ECG changes can be associated with a gradual change in cardiac position and may mimic LV hypertrophy and other structural heart disease [28]. Holter monitoring is indicated in patients with known paroxysmal or persistent arrhythmia such as ventricular tachycardia, atrial fibrillation or atrial flutter or those reporting symptoms of palpitations [5].
Echocardiography: After the ECG, echocardiography is the most common investigation for cardiac function. The modality does not involve exposure to radiation (it is based on ultrasound) and can be repeated as often as needed throughout pregnancy [5]. Echocardiography is able to assess the hemodynamic changes noninvasively, thus it is widely used to measure cardiocirculatory indexes during pregnancy and after delivery such as increases in LV end diastolic/systolic dimensions [64]. In the late 2nd and 3rd trimester, the LV assumes a more globular shape with a drop in LV longitudinal function and strain because of the late rise in afterload due to increase in SVR [65,66]. Pregnancy-related changes affect the severity of valvular lesions on echocardiography assessment. When measuring valve regurgitation, it is important to recognize that extra volume load and heart rate in pregnancy can cause an increase in tricuspid regurgitation without any change in valve function [8]. With no standardized measures, it is important for cardiologist to take serial scans and look for trends in valve pathology and analysed together with clinical assessment [28].
Other imaging tests: Cardiac magnetic resonance imaging (MRI) may be useful for diagnosing complex heart diseases and/or pathologies of the aorta [67]. It is recommended when other diagnostic imaging modalities such as transthoracic or transesophageal echocardiography produce findings that do not support a definitive diagnosis. Despite limited available MIR data, the modality is probably safe especially after the first trimester. However, since gadolinium is assumed to breach the foetal blood-placental barrier and the long-term risk of the developing foetus to exposure to gadolinium ions remains unknown, its use should be avoided during pregnancy [68,69]. Computed tomography (CT) is usually unnecessary in the diagnosis of HFP. Since it involves ionising radiation, its use should be avoided during pregnancy. An exception is indication for CT for accurate diagnosis or definitive exclusion of pulmonary embolism [70]. Due to ionising radiation, cardiac catheterization is also not recommended during pregnancy, but if required such as in the case of emergency pacing, radiation dose should be minimized as much as possible and radiation shielding should be used across the abdomen to reduce foetal exposure [70]. Similarly, chest radiograph should be avoided during pregnancy. However, it is only recommended when other imaging modalities not using ionising radiation fail to determine the cause of dyspnoea, cough or other symptoms [71].
Exercise testing: Prior to and during pregnancy, cardiopulmonary exercise testing is useful for objective evaluation of functional capacity, BP response and exercise-induced arrhythmias. It is an important part of the follow-up program of adults with CHD and those with asymptomatic valvular heart diseases [72,73]. The ESC guidelines of CVD in pregnancy recommends exercise testing in women with known heart disease prior to pregnancy to assist in risk assessment. Submaximal exercise tests should reach 80% of the predicted maximal heart rate in asymptomatic patients with suspected CVD. Semi-recumbent cycle ergometry is the most comfortable modality but treadmill walking or upright cycle ergometry are also useful [28]. Stress echocardiography using cycle ergometry may provide additional diagnostic specificity in detecting the presence and the extent of ischaemia in high-risk patients with possible coronary artery disease. Stress echocardiography may also be useful preconception for assessing myocardial reserve in patients with prior PPCM and recovered LV function as well as in patients with other cardiomyopathies, valvular or CHD [72,73].
Clinical management: Clinical management of HFP largely relies on the individual assessment of a patient’s symptoms and hemodynamic changes to select the most appropriate treatment strategy. The ESC further recommends that clinical management of HFP should consider physiological changes occurring during pregnancy that can affect absorption, excretion and bioavailability of drugs. Changes such as increased intravascular blood volume may require higher dosage of drugs to achieve therapeutic plasma concentrations and dose adaptation needed during treatment. Raised renal perfusion and hepatic metabolism increase drug clearance. Altered pharmacokinetics of drugs also vary in magnitude during different stages of pregnancy requiring careful monitoring and dose adjustment [28].
Pharmacotherapy: Pharmacological therapy of pregnant women with cardiac lesion including management of HFP and the possible maternofoetal effect of medication have been recently published in dedicated book series of Current Cardiovascular Therapy titled, “Cardiac Drugs in Pregnancy”. Depending on the severity of symptoms and the risk to mother’s life, traditional HF medication maybe indicated in some women with HFP [74].
Diuretics are usually the first line of medical therapy for most women with HFP. Although there are concerns that diuretics might reverse or limit normal pregnancy-related physiological changes, there is no evidence that this drug is an independent risk factor for limiting foetal growth. Using diuretics in symptomatic HFP due to increased preload complicated by LV dysfunction may be justified as first line pharmacotherapy. Diuretics therapy may also be uses in HFP when ventricular dysfunction is the cause of PO [75]. On the other hand, combined arterial and venous vasodilator such as nitroglycerin may be beneficial in increasing venous capacitance in acute HFP women with hypertension caused by preeclampsia complicated by both LV systolic failure and renal failure [28].
Angiotensin converting enzymes – inhibitors (ACE-I) and angiotensin receptor blockers (ARBs) intercept the renin-angiotensin-aldosterone axis resulting in natriuresis, decreased intravascular volume and vasodilation. These medications are standard form of treatment in non-pregnant HF patients but in pregnant women in a state of hypereninism, these drugs are contra-indicated [4]. Besides the effect of ACE-I and ARBs on the renin-angiotensin-aldosterone axis in regulating central hemodynamics, they may also be useful in controlling specific perfusion of the uterus and kidney, and thus, are again contraindicated because they increase the risk of babies delivered with neonatal renal failure [76,77].
Beta-adrenergic receptor blockade (beta-blockers) slows the heart rate to allow greater filling during diastole. It improves symptoms and increases survival in patients with systolic dysfunction and depressed ejection fraction < 40% [78]. However, the use of beta-blockers in hypertensive pregnant women may limit intrauterine growth and increase perinatal mortality. Nevertheless, beta-blocker may be indicated in pregnancy when the mother’s life is at risk and when both maternal cardiac disease and treatment can cause adverse perinatal outcomes [79]. At present, the evidence on the risks associated with beta-blocker are confounded by the inability to differentiate between perinatal effect of the disease and treatment effects.
Spironolactone is a potassium-sparing aldosterone antagonist with a mild diuretic effect. It has a synergistic effect with other diuretics resulting in significantly decreased risk of mortality in non-pregnant HF patients [80]. However, its use is contraindicated in HFP because of its anti-androgen effect and some evidence of teratogenesis in a rat-model [4]. During the puerperium, medical therapy of the mother should proceed according to the needs of the adult since spironolactone results in < 1% of the drug passing from mother to child via the breastmilk [28].
Bromocriptine may be indicated in HFP women to suppress lactation and prolactin secretion. In HFP due to PPCM, one of the pathophysiologic mechanism is the presence of 16 kDa cathepsin-cleavage product of prolactin, which has apoptotic effects on the myocardium [41]. Because PPCM has poor prognosis, bromocriptine therapy may be used to suppress lactation but current evidence is inconclusive. Nonetheless, a multi-centre randomized trial is in underway to investigate the effect of bromocriptine on LV function in women with PPCM [81].
Percutaneous therapy: Percutaneous therapy is not recommended but if absolutely necessary, the optimal time is after the fourth month in the second trimester. By this time organogenesis is complete, foetal thyroid is inactive and uterine volume still small and consequently, there is a larger distance between foetus and maternal chest than in later months. Time for fluoroscopy and cineangiography should be as brief as possible and uterus shielded from direct radiation. Heparin is given targeting activated clotting time ≤ 200 s and < 300 s [28].
Cardiac surgery with cardiopulmonary Bypass: Although maternal mortality during cardiopulmonary bypass is similar to that of non-pregnant women [82], it is associated with increased risk of late neurological impairment in children and foetal mortality, and therefore, rarely indicated. Cardiac surgery is only recommended when optimal medical therapy or other non-surgical interventional procedures are unsuccessful and the mother’s life is at risk [83]. Surgery is recommended from 13th to 28th week of pregnancy to prevent the risk of foetal malformations in the first trimester, or preterm delivery and maternal complication in the third trimester. Since at 26 weeks, foetal survival is approximately 80% with 20% having serious neurological impairment, caesarean delivery may be considered prior to cardiopulmonary bypass if gestational age is > 26 weeks but when gestational age ≥ 28 weeks, delivery before surgery should be considered [84].
Treating reversible causes: Reversible diseases that may cause or aggravate HFP include anaemia, overt or occult infection and hyperthyroidism. These diseases are reversible and should be managed on their own merit. In particular, hyperthyroidism in the puerperium is often subclinical and usually under-diagnosed yet it may affect up to 5% of women [85]. Other infections such as urinary infection, frequently leads to sepsis during pregnancy and in the setting of the risk of genital tract sepsis after delivery requires identification and prompt treatment [28].
Meta-analysis of diagnosis and safety of HF medication during pregnancy: Definitive diagnosis of HFP is challenging because signs and symptoms due to pregnancy-related physiological stress mimics that of HF. Diagnosis begins with careful assessment of the patient’s history and physical examination, ECG tests and finally cardiac imaging. In addition to diagnosis of HF, determination of the underlying cardiac disease is important to inform treatment if the cause is reversible [28]. After diagnosis, medical therapy is usually the first-line of treatment. Medical therapy in HFP relies on the traditional HF medication such as diuretics, ACE-I/ARBs and beta-blockers, which have proven safe and efficacious in non-pregnant HF patients. Whereas the safety of diuretics in pregnancy has been demonstrated, evidence on foetal safety after exposure to ACE-I remains inconclusive because of the potential to reverse or limit pregnancy-related hemodynamic changes and reduce uterine and placental perfusion. In the present meta-analysis, we evaluate diagnosis and factors complicating HFP, and the safety of ACE-I therapy on foetal outcomes.
Methods
Study search and inclusion
Online databases PubMed EMBASE and Cochrane were searched for studies investigating the safety of cardiac MRI or HF medication on maternofoetal outcomes from inception to January 2019. Manual search for additional studies was also performed by reviewing references of included studies and relevant review articles and published systematic reviews. The search and eligibility criteria included population in interest (pregnant women with known or undiagnosed cardiac conditions), exposure or interest (cardiac MRI or ACE-I therapy during pregnancy), and outcomes of interest (major foetal malformations). There was no restriction based on language, country, publication year, study design or duration of follow-up. Conference abstracts, case reports and review articles were excluded.
Study selection and data abstraction
Figure 1 shows the process of study selection and inclusion. Two reviewers independently screened all the titles retrieved from online search and bibliography review and then screened abstract and full-texts of all the eligible studies. All the studies that met the eligibility criteria were included in this meta-analysis. Any discrepancy in the inclusion of studies was resolved through consensus. The two reviewers also extracted relevant data from all the included studies. Only information from published data was collected on first author, year, study design, number of participants, mean age, HF medication used and foetal outcomes (Tables 5-6).
Table 5. Study design, patient characteristics and outcomes/complications (CHF: Congestive Heart Failure; PH: Pulmonary Hypertension; PO: Pulmonary Oedema)
| 1st author (year) [Ref #]
|
Study design, country year
|
No. of patients
|
Mean age
|
Patient selection criteria
|
Outcomes/ complications
|
Siu (2001) [21] |
Prospective cohort, Canada, 1994-1999 |
562 |
28±6 |
Pregnant women with congenital or acquired cardiac lesions or cardiac arrhythmias |
Pulmonary oedema, arrhythmia, stroke, or cardiac death in 13% of pregnancies |
Langford (2009) [86] |
Prospective Cohort, London UK, 2004-2007 |
103 |
NR |
Referred pregnant women with known or suspected cardiac disease |
Cardiac symptoms/signs without known heart disease are unlikely to represent high-risk cardiac disease |
Nqayana (2008) [87] |
Retrospective cohort, South Africa, 2007-2008 |
95 |
21-25 |
Pregnant women admitted with cardiac disease |
Cardiac disease in pregnancy is associated with high maternal morbidity and adverse foetal outcomes |
Yaghoubi (2013) [88] |
Cross sectional, Iran, 2007-2012 |
200 |
29±4 |
Pregnant women with significant cardiac diseases admitted for labour at > 28 weeks gestation |
Prolonged hospital stay, maternal and neonatal mortality |
Indira (2015) [89] |
Prospective cohort, India, 2006-2007 |
60 |
NR |
Women with cardiac diseases complicating pregnancy |
Pregnancy complications included congestive HF, PH, atrial fibrillation and PO |
Ruys (2014) [90] |
Retrospective cohort (ROPAC) 2007-2011 |
1321 |
30±6 |
Pregnant women with suspected structural heart diseases |
HF is common at end of 2nd trimester/postpartum, resulting from PH, cardiomyopathy, preeclampsia |
Yassin (2015) [91] |
Cross-sectional, Sudan, 2011-2012 |
75 |
30±6 |
Pregnant women with heart diseases presenting to the hospital ± complications |
CHF, arrhythmias, pulmonary embolism, and PO |
Sneha (2017) [92] |
Retrospective cohort India, 2014-2016 |
36 |
NR |
Pregnant women with known or suspected cardiac diseases ≥28 weeks gestation |
Sustained tachyarrhythmia or bradycardia followed by PO |
Hossinzadeh (2018) [93] |
Cross-sectional, Iran, 2015-2017 |
90 |
28±9 |
Pregnant women with heart disease presenting in Clinic for Obstetrics |
Dyspnoea, cardiac palpation, preeclampsia and hypertension |
Table 6. Summary of study design, patient characteristics and foetal outcomes (ACE-I: Angiotensin Converting Enzyme – Inhibitor; ARB: Angiotensin Receptor Blockers; NS: Not Stated)
1st author (year) [Ref #] |
Study design |
Country |
Study years |
No. of patients |
Foetal outcomes (congenital, cardiovascular, and/or central nervous system malformations) |
Cooper (2006) [94] |
Retrospective cohort study |
U.S |
1985-2000 |
209 |
ACE-I is associated with increased risk for foetal malformations of the cardiovascular system and the central nervous system |
Caton (2009) [95] |
Retrospective cohort study |
U.S. |
1997-2003 |
5021 |
Antihypertensive medication or the underlying hypertension may increase the risk of having an infant with left/right obstructive and septal defects |
Lennestal (2009) [96] |
Retrospective cohort study |
Sweden |
1995-2006 |
1418 |
Antihypertensive therapy had little effect on foetal cardiovascular malformations. |
Li (2011) [97] |
Retrospective cohort study |
U.S. |
1995-2008 |
755 |
Compared to hypertensive controls, ACEI in the 1st trimester is not associated with increased risk of congenital heart defects. |
Moretti (2012) [98] |
Prospective, observational, controlled cohort study |
Canada |
NS |
139 |
ACEI/ARBs is associated with significantly lower birth weight, gestational age and miscarriage but no significant differences in malformations |
Bateman (2017) [99] |
Retrospective cohort study |
U.S. |
2000-2010 |
2631 |
In hypertensive women (adjusted for diabetes) ACE-I has non-significant effect on congenital malformations. |
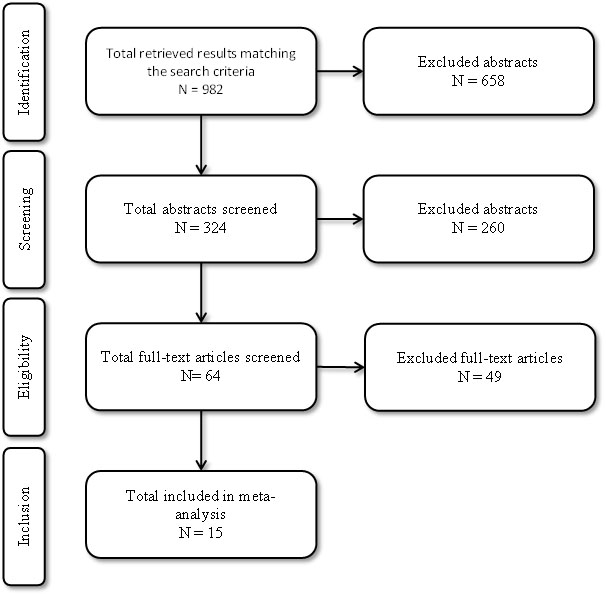
Figure 1. Flow diagram of literature search and inclusion process
Results
Study characteristics
Fifteen (15) studies that met the inclusion criteria were included in this systematic review and meta-analysis [21, 86-99]. In all, the 15 studied enrolled 12,715 patients. Eight studies were retrospective cohort [94-97,99], four were prospective cohort [21,86,89,98] and three were cross-sectional studies [88,91,93]. Nine (9) studies investigated clinical diagnosis of HFP [21,86-93] (n=1,542; age range 21-30 years) from different countries (Canada, U.K., South Africa, Iran, India and Sudan) while the remaining six investigated safety of ACE-I on foetal outcomes [87,90,92,94-99] (n=10,173) from the U.S., Sweden and Canada. Studies on diagnosis of HFP reported underlying cardiac diseases as well as HF signs and symptoms complicating pregnancy while studies on ACE-I treatment reported major foetal malformations – congenital, cardiovascular and central nervous system foetal malformations.
Study outcomes
Diagnosis of HFP shows the common categories of cardiac diseases contributing to HFP are valvular heart disease (58.4%; 95% CI: 35.0-78.5), congenital heart disease (23%; 05% CI; 7.8-51.5), and cardiomyopathies (7.0%; 95% CI: 4.0-12.1) (Figure 2). The main factors either leading to hospital visit or complicating HFP varied across the studies. Common clinical features included pulmonary oedema, pulmonary hypertension and arrhythmia. Arterial fibrillation, preeclampsia, dyspnoea and palpitations were also reported in some patients [90,93]. HFP was also associated with prolonged hospital stay, and maternal/neonatal mortality [86,88] but in pregnant women with cardiac signs and symptoms without existing heart disease are unlikely to represent high-risk cardiac disease or HFP [21].
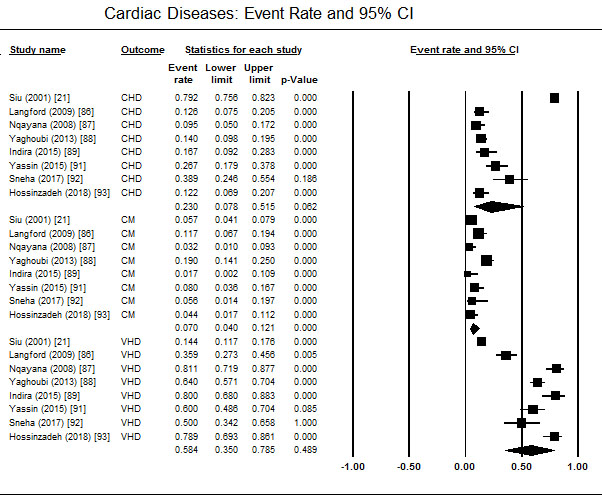
Figure 2. Event rate of cardiac diseases in women with hfp
Treatment with ACE-I in the first trimester showed a tendency towards increased risk for major foetal malformations. In four studies [94-96,99], the risk was significant for cardiovascular malformations (OR 1.51; 95% CI: 1.26-1.80; p = 0.00) (Figure 3) but in two studies each the effect was not significant for congenital malformation (OR 1.79; 95% CI: -.76-4.17; p=0.18) [94,97] (Figure 4) and central nervous system malformations (OR 1.46; 95% CI; 0.19-11.37; p=0.72) [94,99] (Figure 5). However, when exposure to ACE-I is compared to non-hypertensive patients or use of hypertensive, ACE-I has significant risk for congenital heart defects (OR 1.54; 95% CI; 0.90-2.62; p<0.05) [97]. Compared to healthy non-exposed foetus, ACE-I given in the first trimester was also associated with lower birth weight (3225 vs. 3511 g; p<0.001), gestational age (39.6 vs. 37.6 weeks; p<0.001) and miscarriage (18.0% vs. 11.8%; p<0.001) [98].
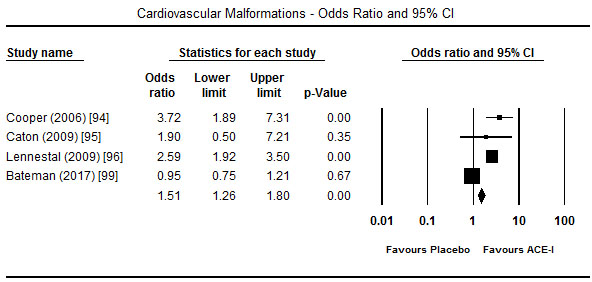
Figure 3. Odds ratio and 95% CI for cardiovascular malformation after ACEI therapy
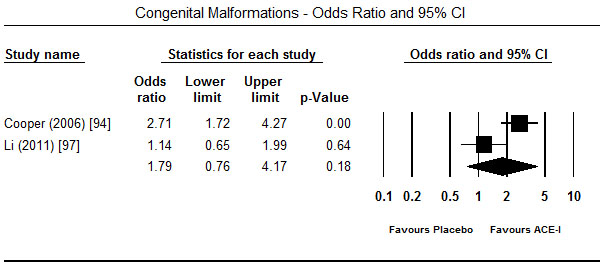
Figure 4. Odds ratio and 95% CI for congenital malformation after ACEI therapy
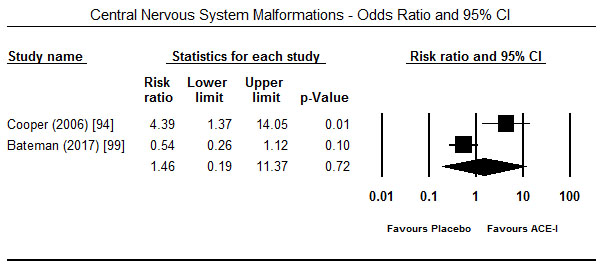
Figure 5. Odds ratio and 95% CI for CNS malformation after ACEI therapy
Discussion of findings
This systematic review and meta-analysis evaluated 12,715 pregnant women with known or undiagnosed cardiac disease including those treated with ACE-I, a traditional HF medication with proven efficacy on non-pregnant HF patients [28]. The results reveal that predominant underlying cardiac diseases that lead to the development of HFP are valvular heart diseases, congenital heart diseases and cardiomyopathy. The major factors complicating HFP as well as associated with unfavourable prognosis include pulmonary oedema, pulmonary hypertension and arrhythmia. The results also reveal that ACE-I administered during the 1st trimester increases the risk of foetal cardiovascular malformation and a tendency towards increased likelihood for congenital and central nervous system foetal malformation in hypertensive pregnant women. ACE-I also increases the likelihood of low birth weight, gestational period and miscarriages compared to healthy non-exposed pregnant women.
The present findings on diagnosis of HFP, which associate underlying cardiac diseases with the development of HFP, are consistent with previous epidemiological studies [11,13] and studies on the normal physiology of pregnancy and the development of HFP [4,8,58,59]. These studies report cardiac diseases limit cardiac ability to tolerate pregnancy-related physiological stress leading to the deterioration of cardiac function as a pump and consequently the development of HFP. Whereas epidemiological studies reveal hypertensive and rheumatic (valvular) heart disease are the most prevalent in pregnancy in developed and developing countries respectively [15,16], the present findings suggest valvular heart disease is the dominant cardiac disease contributing to the development of HFP. However, since most of the included studies were from developing countries (South Africa, India, Iran and Sudan), the findings are consistent with the predominance of valvular heart diseases in pregnant women in developing countries.
The present findings on the treatment of HFP during the 1st trimester suggest a tendency towards increased likelihood for the foetus to develop major cardiovascular, congenital and central nervous system malformations. However, since most of the studies are based on retrospective cohort from national registries combined with the limited number of pregnant women involved in the included studies, the findings would benefit from additional randomized controlled trials to determine the safety of ACE-I to foetus. Although Cooper et al. [94] revealed significant risk for the foetus for major malformations when exposed to ACE-I in the 1st trimester, the study did not control for confounding factors such as the presence of diabetes and hypertension (202 foetus had been exposed to anti-hypertensive treatment). A related study by Malm et al. [100] reported in the absence of diabetes, ACE-I was not associated with a significantly higher teratogenic risk. Moreover, after controlling for hypertension and/or anti-hypertensive medication, Li et al. [97] found an insignificant increase in the risk of congenital cardiac malformations in foetus exposed to ACE-I in the 1st trimester. However, distinguishing the effect of ACE-I on foetus outcomes from that of the underlying cardiac disease continue to limit the understanding of its safety and efficacy on pregnant women. In sum, the present findings suggest that, although the use of ACE-I in the 1st trimester may not be associated with higher risks of foetal malformations, in hypertensive and/or obese patients, ACE-I should be avoided because of increased risk of foetal malformations.
Conclusion
Heart failure (HF) in pregnancy (HFP) describes a new onset of HF during pregnancy and up to six months postpartum not explained by any other known cause. It is prevalent in pregnant women with known or undiagnosed cardiac disease, whose heart cannot tolerate the physiological stress bought about by pregnancy-induced hemodynamic alterations leading to the inability of the heart to function as a pump. It is a leading non-obstetric cause of maternal mortality in both developed and developing countries. Pregnancy risk indices (CARPREG, ZAHARA, WHO and the ESC) list NYHA functional class > II or cyanosis, left heart obstruction, systemic or ventricular dysfunction, decreased sub-pulmonary ventricular function and severe pulmonary regurgitation as the major risk factors for developing HFP. The main pregnancy-specific causes are preeclampsia, peripartum cardiomyopathy and amniotic fluid embolism while non-pregnancy related causes include diseases causing increased vascular resistance (hypertension, pulmonary/congestion and right HF), aortic pathologies (aortic arch syndrome/atherosclerotic disease) and heart disease (cardiomyopathies, valvular diseases and congenital heart diseases). Pregnancy-related hemodynamic alterations that lead to physiological stress include increases in plasma volume, heart rate, cardiac output and stroke volume (1st-2nd trimester) and decreases in blood pressure, stroke volume (3rd trimester) and SVR. Diagnosis depends on careful history and physical examination, risk stratification based on pregnancy indices, electrocardiogram and echocardiogram. If these tests are unclear, additional tests such as magnetic resonance imaging, computed tomography and exercise testing may provide additional diagnostic information. Common therapeutic interventions include pharmacotherapy, percutaneous therapy and cardiac surgery as well as treatment of the underlying cause if reversible. However, the efficacy of pharmacotherapy is unclear because of the difficulty in discriminating maternofoetal effect of treatment from that of maternal cardiac disease.
References
- Anthony J, Sliwa K (2016) Decompensated heart failure in pregnancy. Card Fail Rev 2: 20-26. [Crossref]
- Royal College of Obstetricians and Gynaecologists (2013). Cardiac disease and pregnancy. Good Practice No. 13. [Crossref]
- Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB et al. (2014) Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2: e323-333. [Crossref]
- Pushpalatha K (2010) Cardiac diseases in pregnancy: A review. JIMSA 23: 269-274. [Crossref]
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, et al. (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 62: e147-239. [Crossref]
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, et al. (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37: 2129-2200. [Crossref]
- Hilfiker-Kleiner D, Haghikia A, Nonhoff J, Bauersachs J (2015) Peripartum cardiomyopathy: current management and future perspectives. Eur Heart J 36: 1090-1097. [Crossref]
- Ashrafi R, Curtis SL (2017) Heart disease and pregnancy. Cardiol Ther 6: 157-173. [Crossref]
- Department of Health and Social Security (1989) Report on Confidential Enquiries into Maternal Deaths in England and Wales, 1982–84. Reports on Health and Social Subjects No. 34. London: HMSO; 1989 [Crossref]
- Lewis G, editor (2007) Confidential enquiry into maternal and child health. Saving Mothers' Lives: Reviewing Maternal Deaths to Make Motherhood Safer--2003-2005: the Seventh Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. London: CEMACH. [Crossref]
- Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS, Shackelford KA, Steiner C et al. (2014) Global, regional, and national levels and causes of maternal mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet 384: 980-1004. [Crossref]
- South Africa Department of Health (2015) Saving Mothers 2011-2013: Sixth report on confidential enquiries into maternal deaths in South Africa. Short report, Republic of South Africa Department of Health. [Crossref]
- Mogos MF, Piano MR, McFarlin BL, Salemi JL, Liese KL et al. (2018) Heart Failure in Pregnant Women: A Concern Across the Pregnancy Continuum. Circ Heart Fail 11: e004005. [Crossref]
- Brar SS, Khan SS, Sandhu GK, Jorgensen MB, Parikh N et al. (2007) Incidence, mortality, and racial differences in peripartum cardiomyopathy. Am J Cardiol 100: 302-304. [Crossref]
- Sliwa K, Bohm M (2014) Incidence and prevalence of pregnancy-related heart disease. Cardiovasc Res 101: 554-560. [Crossref]
- Seckeler MD, Hoke TR (2011) The worldwide epidemiology of acute rheumatic fever and rheumatic heart disease. Clin Epidemiol 3: 67-84. [Crossref]
- Elkayam U, Goland S, Pieper PG, Silversides CK (2016) High-risk cardiac disease in pregnancy: Part I. J Am Coll Cardiol 68: 396-410. [Crossref]
- Shime J, Mocarski EJ, Hastings D, Webb GD, McLaughlin PR (1987) Congenital heart disease in pregnancy: short-and long-term implications. Am J Obstet Gynecol 156: 313-322. [Crossref]
- McFaul PB, Dornan JC, Lamki H, Boyle D (1988) Pregnancy complicated by maternal heart disease. A review of 519 women. Br J Obstet Gynaecol 95: 861-867. [Crossref]
- Whittemore R, Hobbins JC, Engle MA (1982) Pregnancy and its outcome in women with and without surgical treatment of congenital heart disease. Am J Cardiol 50: 641-651. [Crossref]
- Siu SC, Sermer M, Colman JM, Alvarez AN, Mercier LA et al. (2001) Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation 104:515-521. [Crossref]
- Khairy P, Ouyang DW, Fernandes SM, Lee-Parritz A, Economy KE et al. (2006) Pregnancy outcomes in women with congenital heart disease. Circulation 113: 517-524. [Crossref]
- Drenthen W, Boersma E, Balci A, Moons P, Roos-Hesselink JW et al. (2010) Predictors of pregnancy complications in women with congenital heart disease. Eur Heart J 31: 2124-2132. [Crossref]
- Sermer M, Harrison DA, Grigoriadis E, Liu G, Sorensen S et al. (1997) Risk and predictors for pregnancy-related complications in women with heart disease. Circulation 96: 2789-2794. [Crossref]
- Roos-Hesselink JW, Ruys TP, Stein JI, Thilén U, Webb GD et al. (2012) Outcome of pregnancy in patients with structural or ischaemic heart disease: results of a registry of the European Society of Cardiology. Eur Heart J 34: 657-665. [Crossref]
- Siu SC, Colman JM, Sorensen S, Smallhorn JF, Farine D et al. (2002) Adverse neonatal and cardiac outcomes are more common in pregnant women with cardiac disease. Circulation 105: 2179-2184. [Crossref]
- Liu H, Huang T, Zhao W, Shen Y, Lin J (2013) Pregnancy outcomes and relative risk factors among Chinese women with congenital heart disease. Int J Gynaecol Obstet 120: 245-248. [Crossref]
- Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cifkova R et al. (2018) 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 39: 3165-3241. [Crossref]
- Balci A, Sollie-Szarynska KM, van der Bijl AG, Ruys TP, Mulder BJ et al. (2014) Prospective validation and assessment of cardiovascular and offspring risk models for pregnant women with congenital heart disease. Heart 100: 1373-1381. [Crossref]
- Thorne S, Nelson-Piercy C, Rosenthal E, MacGregor A, Gibbs S et al. (2006) Pregnancy and contraception in heart disease and pulmonary arterial hypertension. J Fam Plann Reprod Health Care 32: 75. [Crossref]
- Presbitero P, Somerville J, Stone S, Aruta E, Spiegelhalter D et al. (1994) Pregnancy in cyanotic congenital heart disease. Outcome of mother and fetus. Circulation 89: 2673-2676. [Crossref]
- Beauchesne LM, Connolly HM, Ammash NM, Warnes CA (2001) Coarctation of the aorta: outcome of pregnancy. J Am Coll Cardiol 38: 1728-1733. [Crossref]
- Belfort M, Anthony J, Kirshon B (1991) Respiratory function in severe gestational proteinuric hypertension: the effects of rapid volume expansion and subsequent vasodilatation with verapamil. Int J Gynaecol Obstet 98: 964-972. [Crossref]
- Young P, Johanson R (2001) Haemodynamic, invasive and echocardiographic monitoring in the hypertensive parturient. Best Pract Res Clin Obstet Gynaecol 15: 605-622. [Crossref]
- Belfort MA, Anthony J, Saade GR (1993) The oxygen consumption/oxygen delivery curve in severe preeclampsia: evidence for a fixed oxygen extraction state. Am J Obstet Gynecol 169: 1448-1455. [Crossref]
- Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D et al. (2006) Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation 113: 1807-1816. [Crossref]
- Sliwa K, Hilfiker‐Kleiner D, Petrie MC, Mebazaa A, Pieske B et al. (2010) Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail 12: 767-778. [Crossref]
- Nanda S, Nelson-Piercy C, Mackillop L (2012) Cardiac disease in pregnancy. Clin Med 12: 553-560. [Crossref]
- Elkayam U, Goland S, Pieper PG, Silversides CK (2016) High-risk cardiac disease in pregnancy: Part II. J Am Coll Cardiol 68: 502-516. [Crossref]
- Hilfiker-Kleiner D, Sliwa K (2014) Pathophysiology and epidemiology of peripartum cardiomyopathy. Nat Rev Cardiol 11: 364-370. [Crossref]
- Sliwa K, Fett J, Elkayam U (2006). Peripartum cardiomyopathy. Lancet 368: 687-693. [Crossref]
- Ansari AA, Fett JD, Carraway RE, Mayne AE, Onlamoon N et al. (2002) Autoimmune mechanisms as the basis for human peripartum cardiomyopathy. Clin Rev Allergy Immunol 23: 301-324. [Crossref]
- Patten IS, Rana S, Shahul S, Rowe GC, Jang C et al. (2012) Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 485: 333-338. [Crossref]
- Moore J, Baldisseri MR (2005) Amniotic fluid embolism. Crit Care Med 33: S279-S285. [Crossref]
- Blanco MV, Roisinblit J, Grosso O, Rodriguez G, Robert S et al. (2001) Left ventricular function impairment in pregnancy-induced hypertension. Am J Hypertens 14: 271-275. [Crossref]
- Lindheimer MD, Taler SJ, Cunningham FG (2008) Hypertension in pregnancy. J Am Soc Hypertens 2(6):484-494. [Crossref]
- Seely EW, Ecker J (2011) Chronic hypertension in pregnancy. N Engl J Med 365: 439-446. [Crossref]
- Weiss BM, Zemp L, Seifert B, Hess OM (1998) Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996. J Am Coll Cardiol 31: 1650-1657. [Crossref]
- Sharma BK, Jain S, Vasishta K (2000) Outcome of pregnancy in Takayasu arteritis. Int J Cardiol 75: S159-S162. [Crossref]
- Milewicz DM, Dietz HC, Miller DC (2005) Treatment of aortic disease in patients with Marfan syndrome. Circulation 111: e150-e157. [Crossref]
- Sliwa K, Libhaber E, Elliott C, et al. (2014) Spectrum of cardiac disease in maternity in a low-resource cohort in South Africa. Heart 100:1967-1974. [Crossref]
- Watkins H, Ashrafian H, Redwood C (2011) Inherited cardiomyopathies. N Engl J Med 364: 1643-1656. [Crossref]
- Autore C, Conte MR, Piccininno M, et al. (2002) Risk associated with pregnancy in hypertrophic cardiomyopathy. J Am Coll Cardiol 40: 1864-1869. [Crossref]
- Krul SP, van der Smagt JJ, van den Berg MP, et al. (2011) Systematic review of pregnancy in women with inherited cardiomyopathies. Eur J Heart Fail 13: 584-594. [Crossref]
- Basso C, Corrado D, Marcus FI, et al. (2009) Arrhythmogenic right ventricular cardiomyopathy. Lancet 373: 1289-1300. [Crossref]
- Engelfriet P, Boersma E, Oechslin E, et al. (2005) The spectrum of adult congenital heart disease in Europe: morbidity and mortality in a 5 year follow-up period. Eur Heart J 26: 2325-2333. [Crossref]
- European Society of Gynecology (ESG), Association for European Paediatric Cardiology (AEPC), German Society for Gender Medicine (DGesGM), et al. (2011) ESC Guidelines on the management of cardiovascular diseases during pregnancy. Eur Heart J 32: 3147-3197. [Crossref]
- Robson SC, Hunter S, Boys RJ, Dunlop W (1989) Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol 256: H1060-H1065. [Crossref]
- Hunter S, Robson SC (1992) Adaptation of the maternal heart in pregnancy. Br Heart J 68: 540-543. [Crossref]
- Robson SC, Dunlop W, Moore M, Hunter S (1987) Combined Doppler and echocardiographic measurement of cardiac output: theory and application in pregnancy. Br J Obstet Gynaecol 94: 1014-1027. [Crossref]
- Jensen D, Webb KA, O’Donnell DE (2007) Chemical and mechanical adaptations of the respiratory system at rest and during exercise in human pregnancy. Appl Physiol Nutr Metab 32: 1239-1250. [Crossref]
- Fett JD (2011) Validation of a self-test for early diagnosis of heart failure in peripartum cardiomyopathy. Crit Pathw Cardiol 10: 44-45. [Crossref]
- Emmanuel Y, Thorne SA (2015) Heart disease in pregnancy. Best Pract Res Clin Obstet Gynaecol 29: 579-597. [Crossref]
- Cong J, Fan T, Yang X, Squires JW, Cheng G et al. (2015) Structural and functional changes in maternal left ventricle during pregnancy: a three-dimensional speckle-tracking echocardiography study. Cardiovasc Ultrasound 13: 6 [Crossref]
- Savu O, Jurcut R, Giusca S, van Mieghem T, Gussi I et al. (2012) Morphological and functional adaptation of the maternal heart during pregnancy. Circ Cardiovasc Imaging 5:289-297. [Crossref]
- Estensen ME, Beitnes JO, Grindheim G, Aaberge L, Smiseth OA et al. (2013) Altered maternal left ventricular contractility and function during normal pregnancy. Ultrasound Obstet Gynecol 41: 659-666. [Crossref]
- Shellock FG, Crues JV (2004) MR procedures: biologic effects, safety, and patient care. Radiology 232: 635-652. [Crossref]
- Kanal E, Barkovich AJ, Bell C, Borgstede JP, Bradley WG Jr et al. (2007) ACR guidance document for safe MR practices. AJR Am J Roentgenol 188: 1447-1474. [Crossref]
- De Wilde JP, Rivers AW, Price DL (2005) A review of the current use of magnetic resonance imaging in pregnancy and safety implications for the foetus. Prog Biophys Mol Biol. 87: 335-353. [Crossref]
- Iball GR, Brettle DS (2011) Use of lead shielding on pregnant patients undergoing CT scans: results of an international survey. Radiography. 17: 102-108. [Crossref]
- ACOG Committee on Obstetric Practice (2004). Guidelines for diagnostic imaging during pregnancy. Obstet Gynecol 104: 647-651. [Crossref]
- Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE et al. (2010) ESC Guidelines for the management of grown-up congenital heart disease (new version 2010) The Task Force on the Management of Grown-up Congenital Heart Disease of the European Society of Cardiology (ESC). Eur Heart J 31: 2915-2957. [Crossref]
- Diller GP, Dimopoulos K, Okonko D, Li W, Babu-Narayan SV, Broberg CS et al. (2005) Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation 112: 828-835. [Crossref]
- Sliwa K, Anthony J, (eds). Cardiac Drugs in Pregnancy. Springer London; 2014. [Crossref]
- Ter Maaten JM, Dunning AM, Valente MA, Damman K, Ezekowitz JA et al. (2015) Diuretic response in acute heart failure—an analysis from ASCEND-HF. Am Heart J 170) :313-321. [Crossref]
- Irani RA, Xia Y (2008) The functional role of the renin–angiotensin system in pregnancy and preeclampsia. Placenta 29: 763-771. [Crossref]
- Shotan A, Widerhorn J, Hurst A, Elkayam U (1994) Risks of angiotensin-converting enzyme inhibition during pregnancy: experimental and clinical evidence, potential mechanisms, and recommendations for use. American Journal of Medicine 96: 451-456. [Crossref]
- Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB et al. (1996) The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 334: 1349-1355. [Crossref]
- Swan L, Lupton M, Anthony J, Yentis SM, Steer PJ et al. (2006) Controversies in pregnancy and congenital heart disease. Congenit Heart Dis 1: 27-34. [Crossref]
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A et al. (1999) The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 341: 709-717. [Crossref]
- Haghikia A, Podewski E, Berliner D, Sonnenschein K, Fischer D et al. (2015) Rationale and design of a randomized, controlled multicentre clinical trial to evaluate the effect of bromocriptine on left ventricular function in women with peripartum cardiomyopathy. Clin Res Cardiol 1-7. [Crossref]
- Weiss BM, von Segesser LK, Alon E, Seifert B, Turina MI (1998) Outcome of cardiovascular surgery and pregnancy: a systematic review of the period 1984-1996. Am J Obstet Gynecol 179: 1643-1653. [Crossref]
- Chambers CE, Clark SL (1994) Cardiac surgery during pregnancy. Clin Obstet Gynecol 37: 316-323. [Crossref]
- Parry AJ, Westaby S (1996) Cardiopulmonary bypass during pregnancy. Ann Thorac Surg 61: 1865-1869. [Crossref]
- Learoyd DL, Fung HY, McGregor AM (1992) Postpartum thyroid dysfunction. Thyroid 2: 73-80. [Crossref]
- Nqayana T, Moodley J, Naidoo DP (2008) Cardiac disease in pregnancy. Cardiovasc J Afr 19: 145-151. [Crossref]
- Langford EJ, Makharia MK, Langford KS (2009) Cardiac disease in pregnancy: a District General Hospital perspective. British Journal of Cardiology 16: 98-101. [Crossref]
- Yaghoubi A, Mirinazhad M (2013) Maternal and neonatal outcomes in pregnant patients with cardiac diseases referred for labour in northwest Iran. JPMA 63: 1496-1499 [Crossref]
- Ruys TPE, Roos-Hesselink JW, Hall R, Subirana-Domènech MT, Grando-Ting J et al. (2014) Heart failure in pregnant women with cardiac disease: data from the ROPAC. Heart 100:231-238 [Crossref]
- Indira I, Snitha K, Jyothi (2015) Study of pregnancy outcomes in maternal heart disease. Journal of Dental and Medical Sciences, 14: 6-10. [Crossref]
- Yassin K, Elfil AM, Hamid H, Saeed AK, Aziem AA (2015) Epidemiology of cardiac disease during pregnancy in Khartoum Hospital, Sudan. J Women’s Health Care 4: 2167-0420. [Crossref]
- Sneha P, Sarojamma C, Nagarathnamma R (2017) Cardiac disease complicating pregnancy: A tertiary care centre experience. J Med Sci 2: 41-44. [Crossref]
- Hossinzadeh R, Parizad R, Tabrizi MT, Khojasteh ZG, Ainehchi N (2018) Prevalence of Heart Diseases in Pregnant Women Referred to the Heart Diseases Clinic for Obstetrics. Int J Women's Health Reprod Sci 62: 55-58. [Crossref]
- Cooper WO, Hernandez-Diaz S, Arbogast PG, Dudley JA, Dyer S et al. (2006) Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med 354: 2443-2451. [Crossref]
- Caton AR, Bell EM, Druschel CM, Werler MM, Lin AE et al. (2009) Antihypertensive medication use during pregnancy and the risk of cardiovascular malformations. Hypertens 54: 63-70. [Crossref]
- Lennestal R, Olausson PO, Källén B (2009) Maternal use of antihypertensive drugs in early pregnancy and delivery outcome, notably the presence of congenital heart defects in the infants. Eur J Clin Pharmacol 65: 615-625. [Crossref]
- Li DK, Yang C, Andrade S, Tavares V, Ferber JR (2011) Maternal exposure to angiotensin converting enzyme inhibitors in the first trimester and risk of malformations in offspring: a retrospective cohort study. Bmj 343: d5931. [Crossref]
- Moretti ME, Caprara D, Drehuta I, Yeung E, Cheung S, Federico L, Koren G (2012) The foetal safety of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers. Obstet Gynecol Int 2012. [Crossref]
- Bateman BT, Patorno E, Desai RJ, Seely EW, Mogun H et al. (2017) Angiotensin-converting enzyme inhibitors and the risk of congenital malformations. Obstet Gynecol 129: 174. [Crossref]
- Malm H, Artama M, Gissler M, Klaukka T, Meriläinen J et al. (2008) First trimester use of ACE-inhibitors and risk of major malformations. Reproductive Toxicology 1: 67. [Crossref]





