Our aim is to evaluate the evolution of Hemoglobin (Hb) and iron parameters in COVID-19 patients to optimize the therapeutic management of anemia in these patients.
Hb, s-Ferritin, transferrin saturation (TSAT), Haptoglobin, and inflammation markers were recorded. Correlation between parameters was studied using Pearson´s test. Comparison between data at admission and one week after hospitalization was evaluated using t-test for dependent data or Wilcoxon signed rank test. Significance level was fixed as P<0.05.
1,336 in-patients with COVID-19, 56.4% male and 43.6% females with an average age 62.9 years (sd 18.9).
At admission mean Hb was 13.2 g/dL (standard deviation SD 1.6 g/dL), s-ferritin median 508 μg/dL (25-75 interquartiles 460-876 μg/dL) and transferrin saturation mean 19.3 % (SD 8%). All acute phase reactants had raised values. Hb showed a slow and progressive decrease in patients during admission, more marked in patients with severe symptoms and days warded; mean Hb was 12.5 g/dL (SD 1.8 g/dL) in general ward and 11.2 g/dL (SD 2.1 g/dL) at intensive care unit, p<0.05.
Despite the significant inflammatory profile anemia in most patients is not severe, suggesting the use of iron and erythropoietin to prevent severe anemia in those patients.
hemoglobin, SARS-COV-2, covid-19, ferritin, anemia
In recent months coronavirus disease 2019 (COVID-19) has become a global pandemic. In 10-20% of cases it causes severe acute respiratory syndrome, sometimes fatal, with multiorgan failure and death.
Diagnosis of SARS-CoV-2 Acute Respiratory Distress Syndrome is based on a fraction of the partial oxygen pressure with the inspired oxygen ratio is less than 300mmHg, presence of non-cardiogenic pulmonary edema and the need for invasive mechanical ventilation [1-2]. The most representative analytical features found in severe patients are lymphopenia and increased D-dimer (DD), with C-reactive protein elevation (CRP), interleukin 6 (IL6) [3]. Hypertransamisemia is detected in cases of hepatic involvement, as well as elevation of creatinine kinase and myoglobin in patients with myocardial involvement. In critical patients, cytokine storm induces elevation of interleukin 7, interleukin 10, [2,4,5] coexist; the increase of troponin is common.
Studies have proven a decrease in hemoglobin (Hb) in almost 50% of patients [5,6].
An inflammatory anemia can occur in a situation of acute immune activation; this protective mechanism involves a low circulating iron to prevent the virus from invading the organs, while increasing the effectiveness of cellular immunity [7]. The pathophysiology of this anemia related to decreased proliferation of erythropoietic progenitor cells, reduced stimulation of erythropoietin and a decrease in the half-life of erythrocytes [8]. The imbalance of iron homoeostasis in inflammation is due to increased iron retention within the cells of the reticle-endothelial system. These patients have very high ferritin levels, as an acute phase reactant [9-10].
Nevertheless, some studies suggest that hemolysis and erythrocyte structural changes could play the main role in the ferritin elevation [11], and thus the use of iron chelating agents have been proposed [12].
Our aim is to evaluate the evolution of Hb and iron parameters in COVID-19 patients for better understanding the physiopathology of the disease, to optimize the clinical and therapeutic management of these patients.
This is a multicenter observational study in which analytical parameters have been collected from in patients affected by COVID-19 (positive PCR of nasopharyngeal smears) in two hospitals of North Spain at Navarra (Pamplona and Estella) in the period March and April 2020.
Demographic, clinical and analytical data were retrieved from the Hospital Information System and Laboratory Information SystemHIS and LIS systems, Roche®, version 2.5.3.3596
Analytical parameters recorded: Hb, s-Ferritin, transferrin saturation (TSAT), Haptoglobin, CRP, lactate dehydrogenase (LDH), IL-6, DD and arterial Oxygen Saturation (SO2).
Demographic, clinical and analytical variables were summarized using mean and standard deviation or median and interquartile range for quantitative variables and via frequency and percentages in the categorical ones. Association between analytical parameters was computed using Pearson´s correlation coefficient. Comparisons of the analytical and clinical parameters between hospitals was performed using t-test, Anova test, Mann Whitney´s U test or Kruskal Wallis test. Comparison between data at admission and one week after hospitalization was evaluated using t-test for dependent data or Wilcoxon signed rank test. Significance level was fixed as α=0.05. All analyses were carried out using IBM SPSS v.25 software.
The data has been collected identifying the patient with a numerical code, thus respecting the confidentiality of patients (anonymized base). The databases were merged and integrated into Tecnoquality's e-BDIplus quality management software® which has restricted access by individual identification key (licensed program acquired and integrated by the Navarro Health Service) in compliance with the requirements of the General Data Protection Regulation of May 2018, established by the information systems of the Government of Navarra. The authors had access to the database as part of their routine work, using dissociated and anonymized data, with the approval of the Ethics Committee.
Table 1 summarizes the analytical data of 1,336 patients with COVID-19, 56.4% male and 43.6% females mean age 62.9 years (standard deviation (SD) 18.9 years).
Table 1. Basal Values at admission
Variable |
|
Value |
Age (years) |
Mean (sd) |
62.9 (18.9) |
Gender |
Men |
754 (56.4%) |
Women |
582 (43.6%) |
Hemoglobin (g/dL) |
mean (sd) |
13.2 (1.9) |
CRP (mg/L) |
median (IQR) |
46.3 (105.7) |
Glomerular flitration rate |
median (IQR) |
87.2 (24.2) |
LDH (U/L) |
mean (sd) |
338.1 (161.4) |
Ferritin (µg/L) |
median (IQR) |
508.8 (876) |
D Dimer (mg/L FEU) |
median (IQR) |
735.0 (1099.0) |
Iron (µg/dL) |
mediaa (IQR) |
32.5 (37.0) |
Transferrin (mg/dL) |
mean (sd) |
184.8 (57.8) |
Transferrion saturation (%) |
mean (sd) |
19.3 (21.8) |
Cobalamin (pg/dL) |
median (IQR) |
443.0 (238.0) |
IL- 6 (pg/mL) |
median (IQR) |
33.2 (33.4) |
Oxigen Saturation |
mean (sd) |
92.3 (4.6) |
Sd : standard deviation; IQR : interquartiles; CRP : C reactive protein; LDH : lactate dehydrogenase; Il-6 : interleukin 6
At admission mean Hb was 13.2 g/dL (standard deviation SD 1.9 g/dL), s-ferritin median 508 ng/dL (25-75 interquartiles 460-876 ng/dL) and transferrin saturation mean 19.3 % (SD 8%).
All acute phase reactants showed high values.
The correlation of s-Ferritin with the other acute phase reactants was poor, positive correlation was found for CRP, IL-6, LDH and TSAT (Table 2).
Table 2. Relation between variables
|
R |
p-value |
Hb-Ferritin |
-0,071 |
0.804 |
Hb- SO2 |
-0,008 |
0.571 |
Ferritin-CRP |
0,227 |
<0.001 |
Ferrtitin-IL6 |
0,243 |
0.348 |
Ferrtin-LDH |
0,514 |
<0.001 |
Ferrtin-Transferrin Saturation |
0,105 |
0.056 |
R: correlation coefficient; Hb: hemoglobin; SO2: Oxygen saturation; CRP: C reactive protein; IL-6: interleukin 6; LDH: lactate dehydrogenase.
Haptoglobin was analyzed in patients with Hb < 10.0 g/dL and high ferritin; the mean value was 310 mg/dL, so hemolysis was discarded.
Mean Hb at admission was 13.2 g/dL, 51.2 % of males had Hb<13.0 g/dL 51.9% in females had 12.0 g/dL
493 patients were warded after 1 week from admission; the evolution presented a significant decrease of acute phase reactants and increase in D dimer (marker of fibrin degradation and suggesting thrombosis) (Table 3).
Table 3. Evolution of analytical data one week after admission
Variable |
Admission |
1 week |
p-value |
Hb (n=485)1 |
13.0 (2.0) |
12.6 (2.0) |
<0.001 |
Ferritin (n=277)2 |
712.4 (1133.8) |
679.0 (853.3) |
<0.001 |
CRP (n=465)2 |
104.6 (137.0) |
52.4 (65.0) |
<0.001 |
D Dimer (n=338)2 |
720.0 (800.0) |
1027.0 (1577.0) |
<0.001 |
LDH (n=170)1 |
321.8 (121.1) |
268.8 (104.9) |
<0.001 |
1Mean (Standard deviation) t test t
2Median (Interquartiles) Wilcoxon test
Hb: hemoglobin; CRP: C Reactive Protein; LDH, lactate dehydrogenase.
The evolution of Hb showed a slow and progressive decrease in patients with more days of admission, none of the patients suffered a severe anemia (Figure 1).
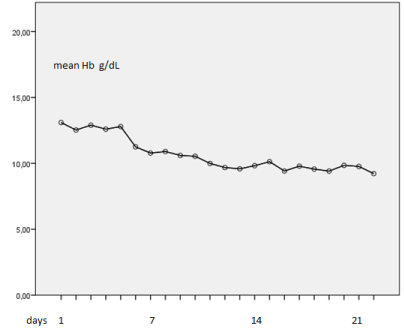
Figure 1. Hemogloblin evolution during stay at hospital
In addition, Hb decreased as the severity of the symptoms increased.
When the patients were divided in those at Intensive Care Unit (ICU), general wards and those in the home hospitalization regime, the mean Hb was 13.6 g/dL (SD 1.6 g/dL) in the latter group, with less hypoxemia and lower level of inflammation (Table 4). In the critical patients admitted ICU with invasive support, the Hb level was lower, mean 11.1 g/dL, compared to 12.7g/dL in patients admitted in general wards with non-invasive oxygen therapy (P<0.001). Likewise, ferritin and DD increase significantly with the severity of disease.
Table 4. Analytical data in different clinical conditions
Variable |
ICU 27.6 % |
General wards
61.4% |
Out patients
11.0 % |
p-valor |
Hb1 g/dL |
11.1 (2.1) |
12.7 (1.9) |
13.6 (1.6) |
<0.001 |
SO22 % |
94.8 (32.7) |
72.3 (23.8) |
- |
<0.001 |
Ferritin3 µg/L |
2055 (1389) |
994 (978) |
591 (591) |
<0.001 |
D Dimer3 (mg/L FEU) |
7269 (2658) |
2847 (994)
2847 |
669 (857) |
<0.001 |
CRP2 mg/L |
6.6 (1.89) |
10.2 (19) |
- |
<0.001 |
1Mean (standard deviation) anova
2Mean (standard deviation) t test
3Median (intequartils) Kruskal Wallis test
ICU: Intensive care unit; Hb: hemoglobin; SO2, Oxygen saturation; CRP: C reactive protein.
Half of the patients with COVID-19 suffered anemia, mean Hb at admission was in reference range. The mean Hb level of the population areas analyzed in this study is not available, however studies in a neighborhood region suggest that this level is similar to that of the non-COVID-19 population with similar average age [13,14].
Considering the mean age in our patients ,63 years, and the fact that Hb tends to decline with age [14-16], we consider that low Hb in patients affected by COVID-19 is not a characteristic of the disease.
Published studies reveal decreased Hb in 40-50% of cases [17]; however, other studies report normal Hb levels with the exception of adolescents with pneumonia [18-19]. A meta-analysis shows that Hb values are essentially reduced in COVID-19 patients with severe disease, compared with mild pneumonia [20], the same trend we found in our patients, with a significantly greater decrease of the Hb in the most severe patients requiring ICU admission compared to the others (Table 4).
This findings can lead to two types of considerations; first , the progressive decrease in Hb suggests that this is a marker of worse clinical progression and the need to maintain Hb levels must be assessed, transfusion support, hematinics and/or erythropoietin administration to prevent the evolution to severe disease; or second, that anemia doesn´t predispose severity, it´s a consequence of clinical course of the disease, taking into account also the iatrogenic component due to multiple analyses (vampirism) [21] and the increase of bleeding risk for the antithrombotic protocols.
In our records the mean Hb progressively decreases, dropping to 10.0 gr/dL in patients who remain warded for two weeks (Figure 1), while Vincent, et al. found similar results after 4 weeks [22]. We consider this primarily an inflammatory anemia, associated to inflammation, as acute phase reactants such as ferritin, CRP and LDH, revealed [9],
In addition, low TSAT reflected an underlying iron deficiency in many patients, while normal level of haptoglobin ruled out the suspicion of hemolysis in these cases. This finding is against studies suggesting possible direct damage of SARS-CoV-2 to the globulin chain causing porphyrin damage with hemolysis and hypoxia [11].
Another important fact is the elevation of inflammatory parameters in these patients. Various publications highlight the consequences of inflammation and anemia in ICU septic patients, the elevation of inflammatory cytokines are associated with low functional iron [23]. The evolution of parameters related to inflammatory anemia (s- erythropoietin, s-ferritin, IL-6 and especially hepcidin) in these critical patients is associated with increased mortality [24]. The results of a study suggest that Hb and ferritin may be biomarkers for identifying disease severity.
SARS-CoV-2 induces the Inflammation-driven increase in hepcidin concentrations which blocks the correct use of iron, increasing ferritin while inducing serum iron deficiency and a decline of Hb [25]. For this reason, anti-inflammatory drugs such as tocilizumab will likely suppress hepcidin synthesis and so increase serum iron [26]. In these patients with such high numbers of inflammatory markers, the level of Hb should be lower than those reported, probably due to the stimulus of hypoxia on erythropoiesis [27], as they are patients with low oxygen saturation. Severe cases of COVID-19 revealed a decreased ratio of arterial oxygen partial pressure to fractional inspired oxygen with hypoxia, tachypnea and hypocapnia (low levels of carbon dioxide) [28]. This hypoxia and hypocapnia in severe cases, could stimulate renal and hepatic erythropoietin and the uptake of iron, improving erythropoiesis, which compensate the inflammatory anemia.
One of the limitations of this study is the lack of s-erythropoietin data that would have allowed us to analyze the relationship with the other parameters studied.
The acute respiratory distress in patients with severe COVID-19 leads to low blood oxygenation levels and can be directly life-threatening because of the body's organs dependence upon adequately oxygenated blood. In case of renal and/or liver failure or severe inflammatory anemia and the production of blood cells cannot compensate the stimulus of hypoxemia allogenic blood transfusion may be necessary to increase tissue oxygenation. It is clinically important to avoid this situation due to its immunomodulatory effect in these patients with an altered immune system. Other treatments used at the beginning of pandemic such as chloroquine and hydroxychloroquine could have a secondary benefit by increasing Hb production an increasing Hb availability for oxygen binding and acetazolamine by causing hyperventilation [29].
The management of anemia requires the evaluation of its etiology to select the appropriate therapy to benefit the patient avoiding the potential harms; based in the experience in warded patients with low Hb and TSAT and inflammatory conditions, the administration of intravenous iron and erythropoietin at limited doses could be the therapy to be applied, closely controlled due to the high thrombotic risk associated [28,30].
- Hb decreases in patients with more days of hospitalization, this trend is more pronounced in severe clinical conditions (ICU).
- No hemolysis has been detected, proven by the stable haptoglobin levels.
- The increase of inflammatory markers, including ferritin, low Hb, low TSAT are compatible with the hypothesized dysregulation in erythropoiesis.
- Despite the significant inflammatory profile with particularly high ferritin numbers, inflammatory anemia in most patients is not severe; TSAT reflects iron deficiency, so the administration of iron and erythropoietin could be the best therapy.
- We must not forget that critical patients have very long stays with blood draws for daily analysis. It is important to exclusively extract the necessary samples, the use micro-samples can aid to avoid iatrogenic anemia.
Authors declare that they have no conflict of interest
No funding was received for this work.
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, et al. (2020) China Medical Treatment Expert Group for COVID-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708-1720.
- Huang C, Wang Y, Li X, Ren L, Zhao J, et al. (2020) Clinica features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497-506.
- Tan L, Kang X, Ji X, Li G, Wang Q, et al. (2020) Validation of predictors of disease severity and outcomes in COVID-19 patients: a descriptive and retrospective study. Med 1: 128-138.e3 [Crossref]
- Wang D, Hu B, Hu C, Zhu F, Liu X, et al. (2020) Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 323: 1061–1069. [Crossref]
- Chen N, Zhou M, Dong X, Qu J, Gong F, et al. (2020) Epidemiological and clinical charcteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395: 507-513. [Crossref]
- Chen L, Liu HG, Liu W, Liu J, Liu K, et al. (2020) Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi 43: E005. [Crossref]
- Brittenham GM, Weiss G, Brissot P, Lainé F, Guillygomarch A, et al. (2000) Clinical consequences of new insights in the pathophysiology of disorders of iron and heme metabolism. Hematology Am Soc Hematol Educ Program 2000: 39-50. [Crossref]
- Weiss G, Goodnough LT (2005) Anemia of chronic disease. N Engl J Med 352: 1011-1023. [Crossref]
- Schroorl M, Snijders D, Schroorl M, Boersma WG, Bartels PCM (2012) Temporary impairment of reticulocyte haemoglobin content in subjects with community-acquired pneumonia. Int Jnl Lab Hem 34: 390-395. [Crossref]
- Muñoz M, Villar I, García-Erce JA (2009) An Update On Iron Physiology. World J Gastroenterol 15: 4617-4626. [Crossref]
- Liu W, Li H (2020) COVID-19: Attacks the 1-Beta Chain of Hemoglobin and Captures the Porphyrin to Inhibit Human Heme Metabolism. ChemRxiv.
- Liu W, Zhang S, Nekhai S, Liu S (2020) Depriving Iron Supply to the Virus Represents a Promising Adjuvant Therapeutic Against Viral Survival. Curr Clin Microbiol Rep 7: 13-19. [Crossref]
- Rivilla Marugán L, Lorente Aznar T, Molinero Rodriguez M, García-Erce JA (2019) Anaemia and the elderly: Critical review of its definition and prevalence. Rev Esp Geriatr Geronto 54: 189-194. [Crossref]
- García-Erce JA, Lorente-Aznar T, Rivilla-Marugán L (2019) Influence of gender, age and residence altitude on haemoglobin levels and the prevalence of anaemia. Med Clin 153: 424-429. [Crossref]
- Freedman ML, Sutin DG (1998) Blood disorders and their management in old age. Brocklehurst Textbook of geriatric medicine and gerontology. (5ª Edn).
- Kulier A, Gombotz H (2001) Perioperative Anämie. Anaesthesist 50: 73-86. [Crossref]
- Lippi G, Plebani M (2020) Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med 58: 1131-1134. [Crossref]
- Henry BM, Lippi G, Plebani M (2020) Laboratory abnormalities in children with novel coronavirus disease 2019. Clin Chem Lab Med 58: 1135-1138. [Crossref]
- Chen F, Liu Z, Zhang F, Xiong RH, Chen Y, et al. (2020) First case of severe childhood novel coronavirus pneumonia in China. Chin J Pediatr 58: 179-182. [Crossref]
- Lippi G, Mattiuzzi C (2020) Hemoglobin value may be decreased in patients with severe coronavirus disease 2019. Hematol Transfus Cell Ther 42: 116–117. [Crossref]
- Ranasinghe T, Freeman WD (2014) 'ICU vampirism' - time for judicious blood draws in critically ill patients. Br J Haematol 164: 302-303. [Crossref]
- Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, et al (2002) ABC (Anemia and Blood Transfusion in Critical Care) Investigators. Anemia and blood transfusion in critically ill patients. JAMA 288: 1499-1507. [Crossref]
- Loftus TJ, Mira JC, Stortz JA, Ozrazgat-Baslanti T, Ghita GL, et al. (2019) Persistent inflammation and anemia among critic III septic patients. J Trauma Acute Care Surg 86: 260-267. [Crossref]
- Jiang Y, Jiang FQ, Kong F, An MM, Jin BB, et al. (2019) Inflammatory anemia-associated parameters are related to 28-day mortality in patients with sepsis admitted to the ICU: a preliminary observational study. Ann Intensive Care 9: 67. [Crossref]
- Cavezzi A, Troiani E, Corrao S (2020) COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clinics and Practice 10: 1271. [Crossref]
- Shah A, Frost JN, Aaron L, Donovan K, Drakesmith H, et al. (2020) Systemic hypoferremia and severity of hypoxemic respiratory failure in COVID-19. Critical Care 24: 320. [Crossref]
- Haase VH (2013) Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev 27: 41-53. [Crossref]
- Geier MR, Geier DA (2020) Respiratory conditions in coronavirus disease 2019 (COVID-19): Important considerations regarding novel treatment strategies to reduce mortality. Med Hypotheses 140: 109760. [Crossref]
- Borges do Nascimento IJ, Cacic N, Abdulazeem HM, von Groote TC, Jayarajah U, et al. (2020) Novel Coronavirus infection (COVID-19) in humans: a scoping review and meta-analysis. J Clin Med 9: 941. [Crossref]
- DeAngelo AJ, Bell DG, Quinn MW, Long DE, Ouellette DR (2005) Erythropoietin response in critically ill mechanically ventilated patients: a prospective observational study. Critical Care 9: R172-R176. [Crossref]
Editorial Information
Editor-in-Chief
Ciro Rinaldi
University Federico II Naples
Article Type
Research Article
Publication history
Received date: December 16, 2020
Accepted date: December 23, 2020
Published date: December 28, 2020
Copyright
©2020 Urrechaga E. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Urrechaga E, Zalba S, Otamendi I, Zabalegui MA, Galbete A, et al. (2020) Hemoglobin and anemia in COVID19 patients. Hematol Med Oncol 5: DOI: 10.15761/HMO.1000217

Figure 1. Hemogloblin evolution during stay at hospital
Table 1. Basal Values at admission
Variable |
|
Value |
Age (years) |
Mean (sd) |
62.9 (18.9) |
Gender |
Men |
754 (56.4%) |
Women |
582 (43.6%) |
Hemoglobin (g/dL) |
mean (sd) |
13.2 (1.9) |
CRP (mg/L) |
median (IQR) |
46.3 (105.7) |
Glomerular flitration rate |
median (IQR) |
87.2 (24.2) |
LDH (U/L) |
mean (sd) |
338.1 (161.4) |
Ferritin (µg/L) |
median (IQR) |
508.8 (876) |
D Dimer (mg/L FEU) |
median (IQR) |
735.0 (1099.0) |
Iron (µg/dL) |
mediaa (IQR) |
32.5 (37.0) |
Transferrin (mg/dL) |
mean (sd) |
184.8 (57.8) |
Transferrion saturation (%) |
mean (sd) |
19.3 (21.8) |
Cobalamin (pg/dL) |
median (IQR) |
443.0 (238.0) |
IL- 6 (pg/mL) |
median (IQR) |
33.2 (33.4) |
Oxigen Saturation |
mean (sd) |
92.3 (4.6) |
Sd : standard deviation; IQR : interquartiles; CRP : C reactive protein; LDH : lactate dehydrogenase; Il-6 : interleukin 6
Table 2. Relation between variables
|
R |
p-value |
Hb-Ferritin |
-0,071 |
0.804 |
Hb- SO2 |
-0,008 |
0.571 |
Ferritin-CRP |
0,227 |
<0.001 |
Ferrtitin-IL6 |
0,243 |
0.348 |
Ferrtin-LDH |
0,514 |
<0.001 |
Ferrtin-Transferrin Saturation |
0,105 |
0.056 |
R: correlation coefficient; Hb: hemoglobin; SO2: Oxygen saturation; CRP: C reactive protein; IL-6: interleukin 6; LDH: lactate dehydrogenase.
Table 3. Evolution of analytical data one week after admission
Variable |
Admission |
1 week |
p-value |
Hb (n=485)1 |
13.0 (2.0) |
12.6 (2.0) |
<0.001 |
Ferritin (n=277)2 |
712.4 (1133.8) |
679.0 (853.3) |
<0.001 |
CRP (n=465)2 |
104.6 (137.0) |
52.4 (65.0) |
<0.001 |
D Dimer (n=338)2 |
720.0 (800.0) |
1027.0 (1577.0) |
<0.001 |
LDH (n=170)1 |
321.8 (121.1) |
268.8 (104.9) |
<0.001 |
1Mean (Standard deviation) t test t
2Median (Interquartiles) Wilcoxon test
Hb: hemoglobin; CRP: C Reactive Protein; LDH, lactate dehydrogenase.
Table 4. Analytical data in different clinical conditions
Variable |
ICU 27.6 % |
General wards
61.4% |
Out patients
11.0 % |
p-valor |
Hb1 g/dL |
11.1 (2.1) |
12.7 (1.9) |
13.6 (1.6) |
<0.001 |
SO22 % |
94.8 (32.7) |
72.3 (23.8) |
- |
<0.001 |
Ferritin3 µg/L |
2055 (1389) |
994 (978) |
591 (591) |
<0.001 |
D Dimer3 (mg/L FEU) |
7269 (2658) |
2847 (994)
2847 |
669 (857) |
<0.001 |
CRP2 mg/L |
6.6 (1.89) |
10.2 (19) |
- |
<0.001 |
1Mean (standard deviation) anova
2Mean (standard deviation) t test
3Median (intequartils) Kruskal Wallis test
ICU: Intensive care unit; Hb: hemoglobin; SO2, Oxygen saturation; CRP: C reactive protein.

