The association of coronary artery disease with hepatitis C has been suggested, but definitive data are still lacking. We aimed to study the association between hepatitis C virus seropositivity and coronary artery disease and to estimate the severity of coronary artery disease in hepatitis C positive patients. The study design is a retrospective case control study. It included two groups of patients with angiographically documented CAD; 100 HCV patients as case group and another 100 non-HCV patients as control group. A detailed qualitative coronary angiographic analysis and Gensini score were used to assess the extent and severity of CAD. The overall results revealed significantly higher Gensini score in the cases group (41.4 ± 30.8) than control group (29.7 ± 21.3), p=0.002. In terms of the number of coronary vessels involved, a distinct pattern with increasing frequency of involvement was noted in the cases group (2.1 ± 1 vs. 1.6 ± 0.7, p<0.001). Regression analysis showed that HCV, old age and DM are independent risk factors for the severity of CAD. The HCV seropositivity might be considered as one of the risk factors affecting the development of coronary artery diseases. There is association between HCV infection and the severity of coronary atherosclerosis as evidenced with a detailed qualitative coronary angiographic analysis, higher Gensini score and increased multivessel affection.
coronary artery disease, coronary angiography, hepatitis C virus
Cardiovascular diseases account for more than 17 million deaths worldwide yearly (around 30% of all global deaths); and this number is expected to rise up to around 24 million by the year 2030. 4 Out of each 5 cardiovascular-related deaths occur in low-income and middle-income countries [1]. In 2010, ischemic heart disease alone caused 7 million deaths worldwide, compared to around 5 million in 1990; with a rise of around 35% [2]. Atherosclerosis is the commonest cause of coronary carotid and peripheral arterial diseases [3,4].
The inflammation hypothesis of atherosclerosis postulates that the critical events involved in the initiation and progression of the lesion are represented mainly by inflammatory and fibroproliferative processes triggered by cytokines and growth factors. However, some infective agents may induce a pro-inflammatory effect and may have a vital role in atherothrombosis [5].
The World Health Organization has declared hepatitis C is a global health problem, with approximately 3% of the world’s population (around 200 million people) infected with HCV. There are about 185,000 new cases a year in the United States alone. In Egypt the situation is even worse; with national prevalence rate of HCV antibody positively of around 10-13%, reaching up to 20% in some areas. The annular incidence of newly infected HCV cases in Egypt is around 7 out of every 1,000 [6-8].
Many factors related to chronic HCV infection are thought to have a role to atherosclerosis. HCV infection stimulates the host immune response, activates T helper cells and releases pro-inflammatory cytokines such as interferon-alpha (IFN-α), interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) [9].
The association between hepatitis C virus (HCV) infection and coronary artery disease (CAD) is controversial. Some studies have reported no association between HCV infection and CAD, whereas others have reported an increased risk. Data have indicated that HCV infection was associated with a higher risk of CAD, after the adjustment of traditional risk factors. Furthermore; HCV infection may independently predict an increased severity of CAD [10]. In the present study, our aim of work is to estimate the severity of coronary artery disease in hepatitis C positive patients and evaluate the association between hepatitis C virus seropositivity and coronary artery disease.
Study design and population
The study design was a retrospective case control study. Using the database in the Qena University Cardiac Catheter Unit we selected 200 patients' data that were subjected to coronary angiography in Qena university cardiac catheter unit during the study period from January 2015 to January 2017. All of the cases had angiographically documented CAD. The patients were divided into two groups. The first group (case group) involving 100 patients with HCV infection and the second group (control group) involving 100 patients without HCV infection. All the patients were investigated for HCV seropositivity by ELISA and proved by PCR. All of the included HCV cases were Child A with compensated liver functions [11,12]. The study has been approved by the ethics committee of Faculty of Medicine, South Valley University, Qena, Egypt.
Inclusion and exclusion criteria
The study included all the patients with more than 50% stenosis in coronary arteries from both sexes and with age of 18 years old or above. We excluded all the patients with less than 50% stenosis, patients with congenital or valvular heart disease, patients with cardiomyopathy, patients with history of interferon administration, patients with HBV seropositivity, patients with decompensated liver diseases, patients with chronic renal failure, patients with history of neoplastic disease and patients with immunological disease.
Methodology
All the cases and controls were subjected to complete history taking, detailed physical examination, 12 leads surface ECG with special emphasis of ischemic changes, laboratory investigation for (random blood glucose level, serum urea, serum creatinine level, HCV seropositivity by ELISA and PCR), coronary angiography in Qena University Cardiac Catheter Unit for detecting of (coronary arteries stenosis, quantitate the severity of the lesion by Gensini score and the number of the coronary arteries affected).
CAD severity was assessed by Gensini score which is based on the percentage of luminal narrowing: 25%: 1 point; 50%: 2 points; 75%: 4 points; 90%: 8 points; 99%: 16 points, and total occlusion: 32 points.
Each coronary lesion score was calculated using percentage of luminal narrowing multiplied by coefficient of coronary segment as shown in Table 1. The Gensini score was calculated by summation of individual coronary segment scores (Figure 1) [13].
Table 1. Coefficient of coronary segments
Lesion site |
Coefficient |
The left main coronary artery (LMCA) |
5 |
The proximal segment of the left anterior descending coronary artery (LAD) |
2.5 |
The proximal segment of the circumflex artery (CX) |
2.5 |
The mid-segment of the LAD |
1.5 |
The distal segment of the LAD, all segments of the right coronary artery (RCA) and the obtuse marginal artery |
1 |
Other segments |
0.5 |
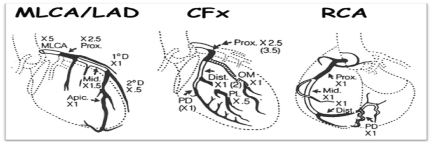
Figure 1. Calculation of Gensini score [13]
Statistical analysis
Data was analyzed using IBM-SPSS for Windows, version 24 (IBM Co., Chicago, USA, 2016). Qualitative data was presented as number and percentage and compared using Chi square test. Quantitative data was presented as mean and standard deviation and was compared using Student t test. In case of Gensinie score, Mann Whitney test was used instead of t test due to non-parametric distribution of data. Binary logistic regression analysis was done to estimate the possible independent risk factors for CAD among out study participants, using either Echo or high Gensini score as the output. P value was considered significant if it was less than 0.05.
Demographic data of the studied patients
In our study there was no difference in the mean age between the two groups as the mean age was 57.1 in the cases group and 57.8 in the control group, Table 2. There was a non-significant difference in the sex distribution between the two groups with p value of 0.298. The number of males in the cases group was 69 (69%) compared to 62 (62%) among controls, Table 2.
Table 2. Comparison between case and control groups according to demographic data and risk factors
|
Cases |
Control |
P. value |
No. |
% |
No. |
% |
Age |
57.1 ± 8.1 |
57.8 ± 6.8 |
0.491 |
Sex |
|
|
|
|
|
Male |
69 |
69.0 |
62 |
62.0 |
0.298 |
Female |
31 |
31.0 |
38 |
38.0 |
Smoking |
46 |
46.0 |
37 |
37.0 |
0.196 |
Diabetes mellitus |
34 |
34.0 |
46 |
46.0 |
0.083 |
Hypertension |
39 |
39.0 |
48 |
48.0 |
0.199 |
Risk factors for CAD among the included patients
The number of smokers among cases was non significantly higher in comparison to the control group with p value of 0.196. The number of diabetic patients was lower in the cases group in comparison to the control group with non-significant p value of 0.083. HTN was not significantly lower among cases compared to controls with p value of 0.199, Table 2.
Electrocardiography data of the included subjects
There was no difference between the two groups as both shows ischemic changes in the ECG, but there was difference in the type of ischemic change in the ECG with p value of 0.017. The most frequent type of ischemia in the ECG in the cases group was inferior ischemia (46%) followed by anterior ischemia (22%). The most frequent type of ischemia in the ECG in the controls group was anterior ischemia (34%) followed by inferior ischemia (27%) (Table 3).
Table 3. Comparison between case and control groups according to site of ischemia by ECG
Type of ischemia |
Cases |
Control |
P. value |
No. (%) |
No. (%) |
|
Anterior |
22 (22%) |
34 (34%) |
0.017 |
Inferior |
46 (46%) |
27 (27%) |
|
Other |
32 (32%) |
39 (39%) |
|
Echocardiography data of the included subjects
- Evidence of ischemia in echocardiography: There was no significant difference between the two groups, Table 4.
- Ejection fraction (EF): There was no significant difference between the two groups, Table 4.
Table 4. Comparison between case and control groups according to echocardiography
|
Cases |
Control |
P. value |
No. |
% |
No. |
% |
|
Evidence of ischemia in echocardiography |
63 |
63.0 |
64 |
64.0 |
0.883 |
E.F% |
58.5 ± 9.2 |
59.4 ± 9.2 |
0.495 |
The severity of the lesion by Gensini score |
41.4 ± 30.8 |
29. 7± 21.3 |
0.001** |
Number of vessels involved |
1 |
34 |
34.0 |
58 |
58.0 |
0.002** |
|
2 |
34 |
34.0 |
30 |
30.0 |
|
|
3 |
25 |
25.0 |
11 |
11.0 |
|
|
4 |
3 |
3.0 |
1 |
1.0 |
|
|
5 |
4 |
4.0 |
0 |
0.0 |
|
Mean ± SD |
|
2.1 ± 1 |
1.6 ± 0.7 |
<0.001** |
** Statistically significant difference (p<0.01) |
Severity of the lesion
- Severity of the lesion by Gensini score: The Gensini score was significantly higher in the cases group (41.4 ± 30.8) than control group (29.7 ± 21.3) with a p value of 0.001, Table 4.
- Number of the coronary vessels involved: When the number of coronary vessels involved in the cases and control groups was compared, a distinct pattern with increasing frequency of involvement was noted in the cases group, Table 4.
Regression analysis to predict the possible risk factors for CAD
We used univariate binary logistic analysis for the different possible risk factors taking evidence of ischemia by Echo as an outcome and found that older age was the only risk factor associated with this outcome, Table 5. There was no need to do multivariate regression analysis.
Table 5. Univariate logistic regression analysis taking evidence of ischemia by echo as the outcome
Item |
P value |
Odd's ratio |
CI of Odd's |
HCV |
0.883 |
1.044 |
0.587-1.857 |
Age |
0.010 |
1.054 |
1.013-1.098 |
Male sex |
0.239 |
1.434 |
0.787-2.612 |
Smoking |
0.201 |
1.472 |
0.813-2.664 |
DM |
0.083 |
1.678 |
0.934-3.015 |
HTN |
0.603 |
1.167 |
0.652-2.090 |
However, when we took the severity of CAD using high Gensini score (above the median of 43) as the outcome, we found that HCV, older age and DM were the possible risk factors for this outcome, Table 6. Using multivariate regression analysis for these 3 factors showed that the three factors are independent risk factors for severe CAD, with HCV was the most important factor, followed by age and lastly DM, Table 7.
Table 6. Univariate logistic regression analysis taking high Gensini score (above the median value of 43) as the outcome
Item |
P value |
Odd's ratio |
CI of Odd's |
HCV |
<0.001 |
2.905 |
1.635-5.161 |
Age |
<0.001 |
1.123 |
1.073-1.174 |
Male sex |
0.528 |
1.207 |
0.673-2.167 |
Smoking |
0.233 |
1.410 |
0.802-2.480 |
DM |
0.033 |
1.868 |
1.051-3.322 |
HTN |
0.945 |
1.020 |
0.583-1.784 |
Table 7. Multivariate logistic regression analysis taking high Gensini score (above the median value of 43) as the outcome
Item |
P value |
Odd's ratio |
CI of Odd's |
Rank |
HCV |
<0.001 |
3.827 |
1.971-7.428 |
1 |
Age |
<0.001 |
1.145 |
1.090-1.203 |
2 |
DM |
0.035 |
2.040 |
1.051-3.959 |
3 |
HCV infection is the major cause of chronic liver diseases leading to a wide range of hepatic diseases, including cirrhosis and hepatocellular carcinoma. Moreover, there is an increasing body of data indicating an important role of HCV in the development of many extrahepatic manifestations [14], including accelerated atherosclerosis which can eventually trigger cardiovascular events [15,16].
The major cause of CAD is coronary atherosclerosis (arteriosclerosis), a process that develops as an inflammatory response of the vessel wall to chronic, multifactorial injury and leads to the formation of atherosclerotic plaques in the coronary arteries [17].
Our study was aiming to estimate the severity of coronary artery disease in HCV patients and to investigate the association of hepatitis C virus with angiographically documented obstructive coronary artery disease.
It was noted in our study that the Gensini score was significantly higher in the cases group (41.4 ± 30.8) than control group (29.7 ± 21.3) with a p value of 0.001 and when the number of coronary vessels involved in the cases and control groups was compared, a distinct pattern with increasing frequency of involvement was noted in the cases group (2.1 ± 1 vs. 1.6 ± 0.7, P. value <0.001).
In agreement with our results, Satapathy, et al. [18] study which searched for an association of Coronary artery disease (CAD) with hepatitis C (HCV), it includes a 63 HCV infected patients were compared with 63 age, race, and sex-matched controls without HCV infection undergoing coronary angiography for evaluation of CAD and showed a significantly higher prevalence of CAD among HCV population. Also, this study showed that combined Reardon's severity score was significantly higher among HCV positive group compared to the controls (6.26 ± 5.39 versus 2.6 ± 3.03; respectively). Additionally, significant multivessel CAD was also noted significantly more commonly in the HCV group compared to controls (57.1% versus 15.9%).
Also in agreement with our results, Alyan, et al. [19] was aiming of to determine the effect of HCV infection on the severity of CAD. This study included 139 HCV seropositive and 225 HCV seronegative patients; all having angiographically documented CAD. Both groups were matched as regards traditional risk factors for CAD such as age, sex, hypertension, diabetes mellitus, smoking or family history of CAD. They found that both C-reactive protein and fibrinogen were significantly higher among HCV seropositive patients and also the Reardon severity score was higher (8.75 ± 1.69 among HCV seropositive versus 6.01 ± 1.80 among HCV seronegative cases).
Another studies supporting our results, Vassalle, et al. [20] evaluated whether seropositivity for HCV is associated with the CAD occurrence. The study found that HCV seropositivity to be associated with the presence of CAD (OR=3.2, 95% CI=1.1-9.2). These results were affirmed by Tsui, et al. [21], who found HCV- seropositive patients having higher rates of death, cardiovascular events, and heart failure hospitalizations during follow-up. In addition, these subjects had also significantly lower mean levels of CRP and fibrinogen but higher levels of TNF-α.
In the large study of Butt, et al. [10], which included 82,083 HCV infected and 89,582 HCV-uninfected subjects, HCV infection was also associated with a 1.25fold higher risk of CAD (95% CI=1.2-1.3) despite a favourable risk profile as HCV infected subjects were less likely to have hypertension, hyperlipidaemia, and had lower total plasma cholesterol, LDL-C, and TG levels compared to HCV-uninfected subjects.
In disagreement with our results, Pothineni, et al. [22] reported that patients with active HCV infection have similar angiographic CAD burden like HCV-negative patients as the Number of patients with obstructive CAD was less in HCV group (23% vs 39%, p<0.05) and angiographic Gensini score was similar in both groups. Also the study of Grab, et al. [23] revealed an absence of association of HCV with CAD in patients with rheumatic diseases, however, it may be explained by the small sample size in this study (67 patients with inflammatory rheumatic diseases, 52 patients without IRD and 30 healthy controls).
Many studies support the role of inflammation in the pathogenesis of CAD [24-27]. According to these studies, a complex biochemical process involving many balance disorders between proinflammatory and anti-inflammatory cytokines may be blamed for the initiation, propagation, and even rupture of the atherosclerotic lesions. Some studies shown that this balance disorder; in term of increased proinflammatory markers activity (e.g.tumor necrosis factor α; interleukin 6 and also high sensitivity to CRP) can be detected more among HCV-seropositive subjects, compared with HCV-uninfected control subjects [28,29].
Since inflammation and thrombosis both have critical roles in the pathogenesis of CAD and as HCV infection is associated with some alterations in inflammatory markers, this may explain the possible role of HCV to increases CAD risk. Markers of thrombosis and inflammation have also been associated with more-severe CAD, and the malnutrition inflammation scores are elevated in HCV-infected persons with CAD, compared with those without CAD[19,30].
In conclusion, we have demonstrated an association between HCV infection and the severity of coronary atherosclerosis as evidenced with a detailed qualitative coronary angiographic analysis, higher Gensini score and increased multivessel affection. Our findings suggest that HCV seropositivity might be considered as one of the risk factors affecting the development of coronary artery diseases. This study might be relevant for adding new predictive and prognostic factors to the coronary artery diseases multifactorial entity.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with Ethics committee of the Faculty of Medicine, South Valley University, Qena, Egypt and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. An informed written consent has been obtained from every included patient. Ethics committee’s reference number: 12/2014.
Consent for publication
All patients included in this research gave written informed consent to publish the data contained within this study. If the patient was less than 16 years old, deceased, or unconscious when consent for publication was requested, written informed consent for the publication of this data was given by their parent or legal guardian.
The authors report no conflicts of interest in this work.
The current research has been funded by the authors themselves.
- Thomas H,Diamond J,Vieco A,Chaudhuri S,Shinnar E, et al. (2018) Global atlas of cardiovascular disease 2000-2016: The path to prevention and control. Global heart 13: 143-163.
- Lozano R,Naghavi M,Foreman K,Lim S, Shibuya K, et al. (2012) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet 380: 2095-2128.
- Naghavi M,Libby P,Falk E,Casscells SW, Litovsky S, et al. (2003) From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation 108: 1772-1778.
- Naghavi M,Libby P,Falk E,Casscells SW, Litovsky S, et al. (2003) From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation 108: 1664-1672.
- Shah PK (2001) Link between infection and atherosclerosis: who are the culprits: viruses, bacteria, both, or neither?. Circulation 103: 5-6.
- Miller FD,Elzalabany MS,Hassani S,Cuadros DF (2015) Epidemiology of hepatitis C virus exposure in Egypt: Opportunities for prevention and evaluation. World J Hepatol 7: 2849-2858.
- Amer FA, Gohar M, Yousef M (2015) Epidemiology of hepatitis c virus infection in Egypt. IJTDH 7: 119-131.
- Fallahian F, NajafiA (2011) Epidemiology of hepatitis C in the Middle East. SJKDT 22: 1-9.
- Gershon AS, Margulies M, Gorczynski RM, Heathcote EJ (2000) Serum cytokine values and fatigue in chronic hepatitis C infection. J Viral Hepatitis 7: 397-402.
- Butt AA, Xiaoqiang W, Budoff M, Leaf D, Kuller LH, et al. (2009). Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis 49: 225-232.
- Kok B, Abraldes JG (2019) Child-Pugh classification: Time to abandon?. Semin Liver Dis 39: 96-103.
- Wang J, Wang Q, Yu G, She Q, Zhang W, et al. (2018) Correlation between liver stiffness measured by shear wave elastography and child-pugh classification. J Ultrasound Med 37: 2191-2199.
- GGMD G (1975) The pathological anatomy of the coronary arteries of man, in: e. Gensini GGMD (Ed.), coronary arteriography. Futura Publishing Co, Mount Kisco, New York 271-274.
- Zignego AL, Craxi A (2008) Extrahepatic manifestations of hepatitis C virus infection. Clin Liver Dis 12: 611-636.
- Adinolfi LE, Restivo L, Zampino R, Lonardo A, Loria P (2011) Metabolic alterations and chronic hepatitis C: treatment strategies. Expert Opin Pharmacother 12: 2215-2234.
- Zampino R, Marrone A, Restivo L, Guerrera B, Sellitto A, et al. (2013) Chronic HCV infection and inflammation: Clinical impact on hepatic and extra-hepatic manifestations. World J Hepatol 5: 528-540.
- Buja L, McAllister JH (2007) Coronary artery disease: pathological anatomy and pathogenesis, in: C.J. Willerson JT, Wellens HJJ, Holmes Jr DR, editors (Ed.), Cardiovascular medicine. Springer, London 593-610.
- Satapathy SK, Kim YJ, Kataria A, Shifteh A, Bhansali R, et al. (2013) Higher prevalence and more severe coronary artery disease in hepatitis C virus-infected patients: A case control study. J Clin Exp Hepatol 3: 186-191.
- Alyan O, Kacmaz F, Ozdemir O, Deveci B, Astan R, et al. (2008) Hepatitis C infection is associated with increased coronary artery atherosclerosis defined by modified Reardon severity score system. Circ J 72: 1960-1965.
- Vassalle C, Masini S, Bianchi F, Zucchelli GC (2004) Evidence for association between hepatitis C virus seropositivity and coronary artery disease. Heart 90: 565-566.
- Tsui JI, Whooley MA, Monto A, Seal K, Tien PC, et al. (2009) Association of hepatitis C virus seropositivity with inflammatory markers and heart failure in persons with coronary heart disease: data from the Heart and Soul study. J Card Fail 15: 451-456.
- Pothineni NV, Rochlani Y, Vallurupalli S, Kovelamudi S, Ahmed Z, et al. (2015) Comparison of angiographic burden of coronary artery disease in patients with versus without Hepatitis C infection. Am J Cardiol 116: 1041-1044.
- Grub C, Brunborg C, Hasseltvedt V, Aukrust P, Førre O, et al. (2012) Antibodies to common infectious agents in coronary artery disease patients with and without rheumatic conditions. Rheumatology 51: 679-685.
- Libby P, Ridker PM, Maseri A (2002) Inflammation and atherosclerosis. Circulation 105: 1135-1143.
- Hansson GK, Libby P (2006) The immune response in atherosclerosis: a double-edged sword, Nature reviews. Immunology 6: 508-519.
- Hansson GK (2005) Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352: 1685-1695.
- Fan J, Watanabe T (2003) Inflammatory reactions in the pathogenesis of atherosclerosis. J Atheroscler Thromb 10: 63-71.
- Riordan SM, Skinner NA, Kurtovic J, Locarnini S, McIver CJ, et al. (2006) Toll-like receptor expression in chronic hepatitis C: correlation with pro-inflammatory cytokine levels and liver injury. J Inflamm Res 55: 279-285.
- Rios-Olivares E, Vila LM, Reyes JC, et al (2006) Impaired cytokine production and suppressed lymphocyte proliferation activity in HCV-infected cocaine and heroin ("speedball") users. Drug Alcohol Depend 85: 236-243.
- Elsurer R, Afsar B, Sezer S, et al (2008). Malnutrition inflammation score is associated with coronary artery disease in hepatitis C virus-infected hemodialysis patients. Eur J Clin Nutr 62: 1449-1454.

