Abstract
Background: Hypothalamic Hamartomas (HH) are benign tumors with hyperplastic heterotopic tissue growing in a disorganized fashion. They are associated with refractory epilepsy. The typical seizure type is “Gelastic”. HH can produce different types of seizures. The epileptogenesis has been discussed focusing in “hamartoma- centrism” and “extra hamartoma epileptogenesis”.
Objectives: To determine the pre-surgical aspects and surgical techniques with the best outcomes in HH associated to refractory epilepsy.
Methods: We studied pediatric patients with HH who underwent epilepsy surgery between 2004 and 2017. We analyzed the type of seizures, neurological comorbidity, type of hamartoma in MRI, the electroencephalography records, type of surgery and outcome according to the Engel’s and ILAE’s classification. We also compare the neuropsychological evaluation before and after surgery.
Results: 7 cases fulfilled inclusion criteria, the ages of starting symptoms vary since 4 months to 7 years old; the mean time of surgery was 5 years. 14% of cases had Gelastic semiology as the first seizure, 57% experienced gelastic seizures and 85% of the cases had at least one generalized seizure. 71.4% were classified as wide implantation and 28.6% as pedunculated. 100% underwent open surgery, through trans-callosal technique in 42.85% and 57.14% through trans-silvian surgery. The outcome after surgery, according to Engle classification: 2/7 (28.57%) resulted with Ia; 2/7 (28.57%) were Ic; 1/7 (14.28%) was type II, 1/7 (14.28%) was type III and 1/7 (14.28%) was type IVc.
Conclusions: The possibility to remove the totality of the tumor was 14%. Nonetheless the results evaluated with ILAE’s and Engel’s outcome score were not bad, possibly because the surgery gets the splitting of the hamartoma from the hypothalamus. The 84% are classified with improvement after surgery and 42.85% are currently without seizures. No patient was able to discontinue the AEDs; 57% required increase the doses and the number of medications.
Key words
epileptogenesis, gelastic seizures, hypothalamic hamartoma
Introduction
Gelastic seizures (GS) have been well known as part of the hypothalamic hamartoma (HH) syndrome from sessile HH, while endocrinological disturbances [1-3] and or visual impairment may arise from pedunculated HH [1,4,5]. Compulsive burst of laughter is a typical and classical feature of GS [1,6,7]. Drop attacks and other secondary generalized epilepsy symptoms usually develop as the patient ages. Other types of seizure, cognitive deterioration and behavioral problems follow and frequently develop late in the first decade of life [1,8,9].
It has been well known that epileptic discharge originates from the hamartoma itself and propagates to subcortical and cortical areas.
Partial and generalized seizures result along with cognitive/ behavioral problems, including memory deficit and mental retardation [1,7,10].
The exact physiopathology about the epileptogenesis in HH and the resulted GS is unknown, there are several studies trying to respond this enigma.
In this study we show our experience in the diagnosis and management in HH associated with refractory epilepsy, emphasizing in the results on GS; as well as we review the available literature about epileptogenesis in HH and epileptic networks in GS, focusing in the abnormal association among the cells constituting the HH.
Methods
We analyze the medical records of patients operated in the Pediatric Epilepsy Surgery Program with HH, was collected all the registers between 2004 and 2017. We evaluate different types of variables (some quantitative and other qualitative). Inclusion criteria: 1. Patients under 15 years old, 2. Involved in the epilepsy surgery program for refractory epilepsy, 3. Lesion defined by neuro radiologist as HH, 4. Diagnosed by neuro physician as secondary epilepsy to the HH 6. Presence of Gelastic seizures and other type of seizures, 7. Medical monitoring for at least 2 years after surgery. Exclusion criteria: HH operated for other reasons different to epilepsy. (Precocious puberty, mass causing intracranial hypertension).
We obtained 11 cases operated of HH, 7 of them fulfilled all inclusion criteria. The quantitative results were analyzed using range, mean, mode and median. The qualitative founds were analyzed according to the presence or absence of specific topics defined by the authors. In some cases, was necessary to call the patient to get specific data.
Results
We obtained 7 cases, the ages of starting symptoms vary since 4 months to 7 years old (y.o), average of starting symptoms were 3 years; the mean time of surgery after the diagnostic was 5 years; roughly the patients had 112 seizures per month before surgery. We found 3 patients with more than 30 seizures per month (Table 1).
Table 1. Demographic features
Case |
Age starting symptoms |
Time since the diagnostic to the epilepsy surgery |
Number of seizures/month before surgery |
1 |
2 Years |
11 Years |
30-60 |
2 |
7 Years |
1 Year |
6 |
3 |
3 Years |
10 Months |
15 |
4 |
1 Year |
9 Years |
15 |
5 |
6 Months |
11 Years |
>300 |
6 |
7 Years |
2 Years |
4 |
7 |
4 Months |
7 Months |
60 to 750 |
The first seizure was in the 71% of cases: focal; 29% were generalized. Only the 14% of cases had Gelastic ictal semiology as the first seizure (one case), the maturation of the epileptogenic zone finished yielding secondary generalization in 85% of cases (6/7), only 15% of the patients remained with focal seizures including gelastic type, However 42% (3/7) had gelastic seizures as part of their epilepsy before surgery (Table 2).
Table 2. Type of seizures for patient
Case |
Type of seizures before surgery |
Type of first seizure |
1 |
Type 1: Right Hemitonic seizures (1-2 per day, 1 min long) Type 2: Tonic generalized (1 to 20 min long) until 7 years |
Focal motor seizure of right hemi-body |
2 |
Gelastic seizures |
Gelastic |
3 |
Generalized |
Generalized seizure |
4 |
Focal s. + generalized s. + gelástic s. |
Left hemi-body focal motor seizure |
5 |
Tonic- Clonic Generalized s. |
Tonic clonic generalized |
6 |
Temporal focal seizures and secondary generalized |
Temporal focal seizure |
7 |
Short inspiration followed by tonic attitude on arms |
Short inspiration followed by tonic attitude on arms |
57% of the patients experienced gelastic seizures as part of their epilepsy and 85% of the cases had at least one generalized seizure during their clinic course (Table 3).
Table 3. Presence of Gelastic or Generalized seizures in the patients
Case |
Gelastic Seizures Yes/ No |
Generalized seizures Yes/ No |
1 |
Not |
Yes |
2 |
Yes |
Not |
3 |
Not |
Yes |
4 |
Yes |
Yes |
5 |
Yes |
Yes |
6 |
Yes |
Yes |
7 |
Not |
Yes |
The means of antiepileptic drugs taken by the patients were 2 (usually Carbamazepine: CBZ and Valproic Acid: VPA). One of our patients strikingly was allergic for all kind of medications (Table 4).
Table 4. Preoperative AED in the studied population
Case |
Antiepileptic drugs before surgery |
1 |
Primidone, CBZ |
2 |
CBZ, VPA |
3 |
CBZ, VPA |
4 |
VPA, FNB, FNT (allergenic reaction for all AED) |
5 |
FNB, CBZ |
6 |
Oxcarbazepine, VPA |
7 |
LEV, VGT |
Other preoperative symptoms or signs different to epilepsy were classified as those with impact on psychomotor development, endocrinological, visual, behavioral and motor signs of focalization.
57% (4/7) of the patients had normal psychomotor development, 28% had mental retardation and 15% had a psychomotor delay achieving finally the goals.
57% (4/7) had concomitant endocrinological symptoms whether associated to precocious puberty as well as hypopituitarism expressed as secondary hypothyroidism. 75% of the endocrinological disorders were precocious puberty and 25% was hypopituitarism.
One of the patients (14%) had bilateral blindness, the rest did not have any visual abnormalities (86%).
28.6% (2/7) had behavioral disorders usually aggression and rage, 71.4% did not have any complaint in this topic.
No one had focal motor symptoms before surgery as a HH manifestation (Table 5).
Table 5. Other symptoms in HH
Case |
Preoperative Psychomotor Development |
Endocrinological symptoms before surgery Yes/Not |
Visual symptoms before surgery Yes/Not |
Behavioral symptoms |
Focal motor symptoms before surgery Yes / no (Which?) |
1 |
Normal development until 2 y.o. Moderate Mental retardation at 12 y.o |
Yes (precocious puberty) |
Not |
No |
No |
2 |
Normal |
Yes (precocious puberty) |
Not |
No |
No |
3 |
Normal |
Not |
Not |
Yes |
No |
4 |
Severe Mental Retardation |
Yes (hypothyroidism) |
Not |
No |
No |
5 |
Psicomotor Development delay |
Yes (precocious puberty) |
Yes (Bilateral blindness) |
No |
No |
6 |
Normal |
Not |
Not |
Yes |
No |
7 |
Normal |
Not |
Not |
No |
No |
About the features of the HH on images, 71.4% were classified as width implantation and 28.6% as pedunculated; 57% were placed on the intraventricular compartment, 29% extraventricular and 14% occupied both spaces. Respect to the side, the proportion was similar between them: 57% left sided and 43% right sided. No one enhanced with contrast (Table 6).
Table 6. HH and imaging findings
Case |
Imaging feature (sessile/pedunculated/width implantation) |
Intraventricular/ Extraventricula/Both (position respect the third ventricle) |
Right/Left hypothalamus |
Contrast enhacement Yes/Not |
1 |
Wide implantation |
Intraventricular |
Left |
No |
2 |
Wide implantation |
Intraventricular |
Left |
No |
3 |
Wide implantation |
Intraventricular |
Right |
No |
4 |
Pedunculated |
Both |
Rigth |
No |
5 |
Wide implantation |
Extraventricular |
Right |
No |
6 |
Pedunculated |
Intraventricular |
Left |
No |
7 |
Wide implantation |
Extraventricular |
left |
No |
Respect to the volume of the HH, we do not have information in two patients. The mean volume of the lesion was 12 ml. The range was 25.35 ml and the distribution of the values was not modal (Table 7).
Table 7. Size and volume of the HH
Case |
Size (width×length×height) mm |
Volume of the HH (ml) |
1 |
15×11×10 |
1.65 |
2 |
No information |
No information |
3 |
25×15×20 |
7.5 |
4 |
No information |
No information |
5 |
45×20×30 |
27 |
6 |
18×10×13 |
2.34 |
7 |
34,5×16,63×41,04 |
22 |
Electrophysiologically, only 14% had a normal preoperative baseline EEG, 71% of the patients had a slow rhythm mainly with theta waves and this type of slowness was lateralized to the same side of the lesion, most of them were continuous (80%) 20% had a non-continuous theta rhythm. Only 14% had spikes as an abnormal electrographic finding. The most common ictal finding was spikes associated to slow waves and/or poli spikes (86%) only in one patient (14%) there was no found spikes or waves in the surface EEG. The preoperative EEG demonstrated the lobe in 86%, remaining 14% just showed a hemispheric lateralization. Emphasizing in the lobar distribution: 42% had electrographic abnormalities in the frontal lobe, 14.2% in temporal lobe and 28.6% registered frontotemporal abnormalities. In one patient even in the occipital lobe was found ictal activity. The surface EEG demonstrated left hemisphere lateralization in 42.8%, right side in 14.3% and bilateral electrographic signs in 42.8% (Table 8).
Table 8. Electrophysiological findings
Case |
Preoperative Baseline EEG |
Preoperative Ictal electrophysiological findings |
Preoperative EEG Lobar side |
Preoperative EEG lateralization |
1 |
Bilateral Theta and delta waves predominantly on left hemisphere |
Bilateral and multifocal Spike and slow wave of high to middle voltage , more in left hemisphere and in anterior compartment, with periods of cortical inhibition |
Frontotemporal |
Left |
2 |
Asymmetric slowdown, predominantly on Fp1 and F3 |
F3-F7 Spike and polispike, F4 spread |
Frontotemporal |
Left |
3 |
Bilateral spikes predominantly in left frontal lobe |
Bihemispheric epileptiform spiking activity |
Frontal |
Bilateral |
4 |
Non continuous theta polimorphic rhythm |
Sincronic spikes |
Temporal |
Left |
5 |
Diffuse slow signs and bilateral irritative electrography, more in bifrontal areas |
Spike with or without slow wave |
Frontal |
Bilateral |
6 |
Normal |
Spike -slow wave |
Occipital (1999) Frontal (2000) |
Bilateral |
7 |
Diffuse slowdown. Less voltage in right hemisphere |
No epileptiform activity |
Hemispheric |
Right |
The surface EEG was concordant with the side of the HH in the 28.6%, recorded bilateral findings in presence of unilateral HH in 42.8%; localized the opposite hemisphere in 28.6%. (2/7) With regard to the latter, one of the patients recorded the ictal activity on the left hemisphere being the lesion right sided, but the HH in this case had intra and extra ventricular compromise and in the second one, the EEG showed ictal activity in the right hemisphere and the HH was in the left, but the electrographic sign was hemispheric, not lobar (Figure 1).
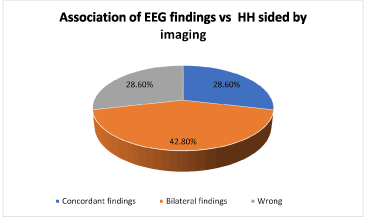
Figure 1. Association of EEG findings vs. HH sided by imaging
About the surgical approach: 100% underwent open surgery, through transcallosal technique in 42.85% and 57.14% through trans-silvian surgery. Only, a patient received radiosurgery. The 85.71% had partial resection. One patient had total resection by trans-silvian approach. Respect to the collateral effects after surgery 42.85% did not have any complication, 57.14% rest, had some kind of adverse neurological deficits post-operative, the most common was the third nerve palsy being the 100% of the patients with complications. One patient suffered a subdural effusion requiring subdural peritoneal shunt, another one had wound infection and 14.28% complained Weber’s Syndrome and Diabetes insipidus. The pediatric ICU stay oscillates between 1 to 3 days (mean: 2.14 days, mode: 2, median: 2, range 2 days) (Table 9).
Table 9. Surgical treatment in HH
Case |
Approach |
Open surgery |
Radio
neurosurgery |
Type of surgery |
Intraoperative side effects |
Postoperative days in ICU |
1 |
Transcallosal |
Yes |
Yes |
Parcial Resection |
Not |
3 |
2 |
Transilvian |
Yes |
No |
Parcial Resection |
Partial third nerve palsy |
2 |
3 |
Transcallosal |
Yes |
No |
Parcial Resection |
Not |
2 |
4 |
Transilvian |
Yes |
No |
Parcial Resection |
Right third palsy nerve and left hemiparesis. Diabetes Insipidus. Hydrocephalus (VPS implanted 1 month postoperative) |
2 |
5 |
Transcallosal |
Yes |
No |
Parcial Resection |
Not |
1 |
6 |
Transilvian |
Yes |
No |
Parcial Resection |
Wound infection and third nerve palsy |
3 |
7 |
Transilvian |
Yes |
No |
Total Resection |
Third nerve palsy, subdural effusion requiring a SPS |
2 |
With respect to the outcome after epilepsy surgery in children with HH in our institution, according to Engle’s classification: 2/7 (28.57%) resulted with Ia; 2/7 (28.57%) were Ic; 1/7 (14.28%) was type II, 1/7 (14.28%) was type III and 1/7 (14.28%) was type IVc. Summarizing 71.42% had improvement after surgery (condition I, II and III) and only 14.28% worsened. Using the ILAE’s classification 3/7 (42.85%) were class 1, 1/7 (14.28%) was class 2, 1/7 (14,28%) was class 3, 1/7 (14,28%) was class 4 and 1/7 (14,28%) was class 6 (Figures 2-4) (Table 10).
Table 10. Postoperative results about seizure control using Engel and ILAE outcome scale and postoperative seizure whether present.
Case |
Postoperative Engel classification |
ILAE outcome scale |
Type of postoperative seizures |
1 |
Ic |
Class 1 |
Only one generalized seizure |
2 |
IVc (3-4 seizures per day) |
Class 6 |
Gelastic,Temporal focal seizures |
3 |
II |
Class 3 |
Gelastic, thrice per day (last evaluation 2011) |
4 |
Ic |
Class 2 |
No seizures just auras |
5 |
IIIc |
Class 4 |
Gelastic + generalized |
6 |
Ia |
Class 1 |
No seizures |
7 |
Ia |
Class 1 |
No seizures |
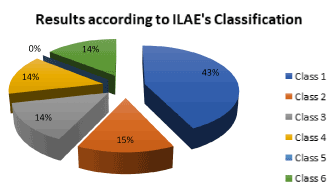
Figure 2. Distribution of the results according to ILAE’s classification
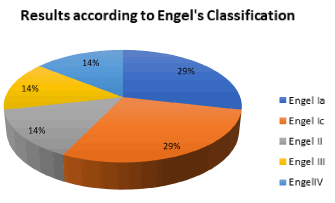
Figure 3. Distribution of the results according to Engel’s classification
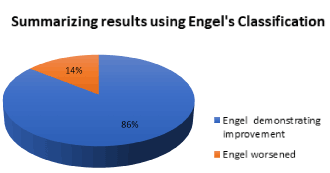
Figure 4. Summarizing results using Engel’s classification
In the postoperative condition, only the patients with Engle’s outcome more than II were performed a control EEG, one of them was demonstrated the presence of right fronto parietal ictal and interictal epileptiform discharges and another one showed generalized spiking.
Analyzing the postoperative medication and with a follow up of only two years and comparing between the number of antiepileptic drugs before and after surgery, we found that 1/7 (14.28%) has maintained the same number of AEDs after surgery, 2/7 (28.57%%) was possible the reduction from 3 to 1 AED and 4/7 (57.14%) required more AEDs post-operative respect before surgery (Figures 5 and 6) (Table 11).
Table 11. Postoperative EEG findings and AED intake
Case |
EEG postoperative |
Postoperative AED |
1 |
No controlled by EEG |
CMZ, VPA, clobazam |
2 |
Right fronto parietal ictal and interictal epileptiform discharges (2016) |
LEV, VPA, clonazepam, lacosamida |
3 |
No controlled by EEG |
VPA, oxcarbacepine, clonazepam |
4 |
No controlled by EEG |
CBZ |
5 |
Generalized sipiking |
CBZ,VPA,Clonazepam |
6 |
Normal |
CBZ |
7 |
No controlled by EEG |
LEV |
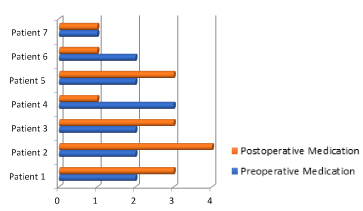
Figure 5. Comparison between preoperative and postoperative medication
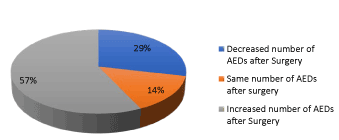
Figure 6. Proportion among the patients who required increase of medication respect to those who were able to decrease it or remain with the same AED
Comparing the neuropsychological development pre and post-surgery, the most of patients remained in the same cognitive condition, only in the patient number 2 was observed some calculation difficulty and in the patient 3 was necessary language therapies after surgery. In one patient the mental development was normal before surgery and in the postoperative suffered intracranial sinus thrombosis. An involution in the mental progression was observed but we cannot assume that the regression occurred for the surgery or for the sinus thrombosis (Table 12).
Table 12. Postoperative Neuropsychological development
Case |
Post-operative Neuropsychological Development |
Observations |
1 |
Moderate Mental retardation |
|
2 |
Specific calculate dificulty |
|
3 |
Normal, the patient is receiving language therapies |
Candidate for radioneurosurgery |
4 |
Severe Mental Retardation |
|
5 |
Moderate Mental retardation |
Patient with Saethre Chotzen syndrome |
6 |
No information |
|
7 |
Psicomotor Development delay |
Nasal Encefalocele, Intracranial sinus thrombosis |
Below we will present a review of the HH focusing in its epileptogenesis
Hypothalamic hamartomas
This rare non-neoplastic abnormal mixture of neuronal and glial tissue of the inferior hypothalamus has been postulated to be derived from the mammilo-thalami-cingulate tract, from which HH is networking other brain areas associated with GS or the pathway from the HH to the brainstem and cerebellum [1,11,12].
Using a recent electroencephalography-functional MRI (EEG-fMRI) studies revealed that the ipsilateral hypothalamus is associated with activation of brainstem tegmentum and contralateral cerebellum; in contrast the cunei, bilateral thalami, caudate nuclei, hippoccampi, paracentral gyri and default mode network (precunei and inferior lateral parietal lobes) are deactivated [1,13].
This activated-deactivated networks result in epileptic discharges through the premotor and frontal opercular areas (voluntary system), which are located on the same side of the broader attachment [1,14] of the HH on the hypothalamus, as well as the ipsilateral thalamus is activated, mainly VA nucleus with the cingulate gyri and the limbic circuit is activated too leading to DM activation (dorso medial thalamic nucleus) with the subsequent “on” activity of orbitofrontal cortex, mesial part of the premotor area and parietal lobe [15] (Figure 7).
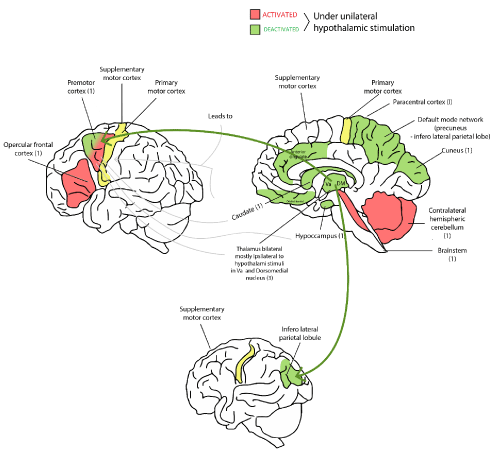
Figure 7. Scheme that represents the activation and deactivation brain areas after discharge initiated into the hypothalamus, in red are depicted the areas with positive input and in green those with negative input. The premotor cortex has both type of stimulus.
This system conveys discharges through the motor cortex, and pyramidal tract to the brainstem [1,14,16].
Intra lesional (inside HH) recordings have revealed that the internal epileptiform discharges mix with low voltage activities [1,10,17-19]. Most epileptiform discharges arise from the broad attachment side [1,18,19].
HH tissue consists of two types of neurons [1,20-23]:
- Small (90% of all HH neurons) à express glutamic acid decarboxylase à deliver GABA
- Large (10% of all HH Neurons) à γ-aminobutyric acid responsive neurons
Platinum micro wire intraoperative recordings of single neuron activity captured 10-20 spikes/sc in over 200 HH neurons, producing synchronous firing due to fast firing neurons [1,24]. Therefore, concomitant scalp EEG and depth electrode recording inside HHs may be very useful in disconnecting this lesion from intractable epilepsy. The depth electrode will guide the disconnection surgery as well as confirm the successful disconnection [1,18,25].
Based on their shape and relationship to the hypothalamus, there are several classifications of HHs, and the symptoms and severity depend on their dimensions (size, localization, type of attachment, degree of hypothalamic displacement) (Table 13) [1,5,26,27].
Table 13. Type of Hypothalamic Hamartomas according to implantation
Sessile type |
Epilepsy
Behavioral disturbances
Hormonal problems |
Pedunculated |
Precocious puberty [1,5, 26, 27] |
Gelastic seizures in patients with HH
The HH is a developmental malformation that occurs in the region of the tuber cinereum and inferior hypothalamus. The epileptic syndrome in HH patients is characterized by laughing (gelastic) seizures beginning in early infancy [27-29]. They are usually refractory to medical treatment, even to ketogenic diet and vagus nerve stimulation [1,3,30].
Although the HH is a benign tumor and the accompanying gelastic seizures often present during the neonatal period, patients later develop additional seizure types [8,28,29].
The most effective treatment for epilepsy associated with HH is surgical resection of the lesion [28,8,31].
Gelastic seizures associated with HH represent the most common disease model of human subcortical epilepsy. These are originated from the “voluntary system” from premotor and frontal opercular areas. Since ictal laughter associated with HH appears mechanical and unnatural [16,28,29] and usually does not result in feeling positive emotions [10,28,32,33].
Initiated by abnormal electrical activity spreading rostrally and dorsally to the areas in the neighboring limbic system, and caudally to the brainstem, the HH can induce physiological and psychophysiological manifestations of laugh attacks [28,34].
Neuropathology of HH
As was said before, small round soma and bipolar or unipolar morphology with largely unbranched and aspiny dendrites [20-22,28] are approximately 90% of total neuron population. These express glutamic acid decarboxilase (GAD) hence, these neurons are GABAergic interneurons with local network connections [23,28,35] (Figure 8).
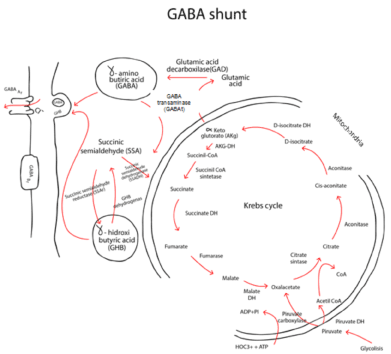
Figure 8. Glutamic acid decarboxylase (GAD) is an enzyme in charge of the metabolism of Glutamic acid to GABA. Here, we depict the relationship among the different enzymes associated to the production of neurotransmitters and the Krebs’s cycle.
Large neurons make up approximately 10% of total neuron population in HH tissue and are dispersed throughout the tissue, although often situated at the edges of the small neuron cluster [20,21,28]. These cells have features consistent with projection-type neurons, including pyramidal morphology, abundant Nissl substance, and multiple processes, often with a single large proximal dendrite [2-22,28]. Dendrites are more likely to be branched and spiny. At least some HH large neurons express markers consistent with excitatory neurotransmitters, such as Vglut2.
Epileptogenesis of human gelastic seizures
The mechanisms underlying the epileptogenesis of gelastic seizures are unknown, the hypothesis available are:
- Local irritation of adjacent normal structures
Especially large size tumors mechanically affect and distort local anatomy, altering the excitability of hypothalamic networks.
Anatomical analysis showed that HH lesions within the third ventricle are often associated with gelastic seizures [4,5,27,28,36] and larger lesions have the features of symptomatic generalized epilepsy [28,37].
However, the presence of epilepsy in patients with HH appears to correlate with the region of lesion attachment (posterior hypothalamus in the region of the mammillary body) rather than lesion size [28,38-40].
- Abnormalities of hormone expression
HH cells express neuropeptides, which may contribute with seizures in other conditions [23,28,41]. Hormones secreted by the hypothalamus and pituitary have been identified during gelastic seizures [3,28].
- HH are intrinsically epileptic
Ictal EEG recordings from electrode contacts placed directly into HH tissue identify seizure onset within the lesion itself [10,28,42]. Direct stimulation of electrodes within HH can evoke typical gelastic seizures [28,34].
Roles of intrinsic neuron firing in seizure genesis
In cortical dysplasia (CD), cytomegalic neurons (but not balloon cells) act as epileptic “pacemakers” and likely contribute to seizure genesis [28,43,44]. Electrophysiological microelectrode recordings have demonstrated that small HH neurons have intrinsic pacemaker-like firing behavior [22,23,28,35,45] (Table 14).
Table 14. Comparison between small and large neurons in HH [*This activity was blocked by tetradotoxin (voltage dependent sodium channel blocker) but no change was seen with GABAA antagonist or inotropic glutamate receptor antagonist, suggesting intrinsic firing pacemaker]
|
Small neurons |
Large neurons |
Neurotransmitter |
GABA |
Glutamic acid |
Soma size |
<16 μm |
>16 μm |
Frequency of spontaneous action potential (firing) |
10.5±0.8 Hz |
Even quiescent at rest (1 Hz) |
Resting membrane potential |
-53.2 ±1.1 mV |
-60.8±1.7 mV |
Regular firing |
63% of neurons |
-- |
Irregular firing |
28% of neurons |
-- |
Burst firing |
*9% of neurons |
-- |
GABA-mediated excitation during normal development in epilepsy
GABA is the most important inhibitory neurotransmitter in the CNS, exhibiting binding ligand-gated (GABAA and GABAC) and G-protein-coupled (GABAA) receptors. GABAAR (receptor) is highly permeable to chloride ions, and its activation tends to hyperpolarize the membrane potential through the influx of these negative ions [28,46,47]. Deficits in GABAA receptor-mediated inhibition are a major mechanism contributing to seizure genesis in several forms of epilepsy [28,48]. In contrast to the hyperpolarizing (inhibitory) effect on normal mature neurons, GABA can exert an excitatory role on immature neurons [28,49,50] (Figure 9).
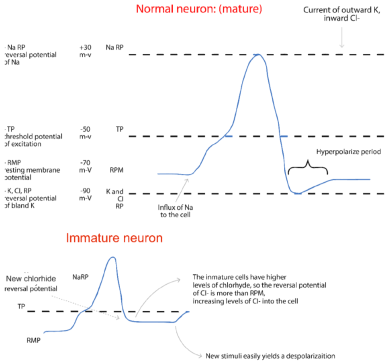
Figure 9. Comparison between a normal neuron and its action potential with an immature neuron, which has abnormalities in the phase 4 in action potential owing to the higher intracellular levels of chloride
If on these immature cells act a GABAA agonist, the Cl- levels inside the cell will increase and the reversal potential of Cl- will be caught soon favoring new excitation (Figure 10).
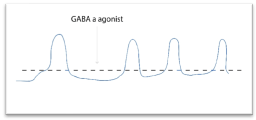
Figure 10. Mechanism through the cell could be transformed to potential exci pacemaker
Ben Ari et al. reported that activation of GABA receptors on immature neurons in neonatal hippocampal slices induced membrane depolarization [28,51]. In these immature neurons, the reversal potential for Cl- was more positive than the resting membrane potential, suggesting that the intracellular Cl- concentration was higher in these neonatal neurons compared to mature neurons. GABA produces depolarization and increased concentration of intracellular calcium in immature neurons in a variety of brain regions including: hippocampus, neocortex and hypothalamus.
Notably, GABAergic excitation is also observed in several highly selected circumstances in normal mature neurons [28,50,52,53]. Functional alteration of GABAA receptors have demonstrated that this functional switch can also been found in some epileptic conditions [28,54].
Therefore, seizures appear to reduce the ability of neurons to prevent excess chloride accumulation, which in turns results in a quasi-permanent accumulation of Cl- intracellulary, leading to the excitatory effects of GABA.
Role of GABAA receptor-mediated excitation in HH epileptogenesis
Most large HH neurons have a functionally immature response with depolarization and increased firing when exposed to GABA agonist [22,28,36,45,50].
Wu et al. studied [28,45] the large and small HH neurons and muscimol was applied (selective GABAA receptor agonist, responsible for psychotropic effects of the fungi Amanita muscaria) resulted in depolarization of 83% of large HH neurons and 24% of small neurons (Figure 11).
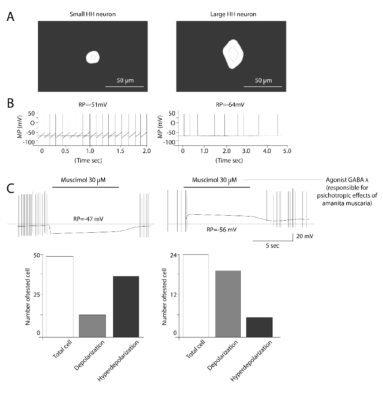
Figure 11. Activation of GABAa Rs differently modulates small and large HH neuron activity. Typical small (A left panel) and large (A right panel) from human HH tissues are depicted using phase contrast photo picture. Both types of HH neurons showed spontaneous AP firing but large cell had much lower firing rate (B) Gramicidin-performed patch-clamp recordings under current-clamp mode demonstrated that Muscimol hyperpolarized small (C left panel) but depolarized large (C right panel) HH neurons. (D) Summary of the numbers of small and large HH neurons and their responses to the GABAaR agonist muscimol using gramicidin-performed recordings. Taken from Wu J, et al. [31]
The functional difference between large and small HH neurons correlates with differences in intracellular chloride concentrations, consistent with reversal potential of Cl- in HH large neurons: Table 15 [28,45,55-57].
Table 15. Large and small neurons: functional differences [*They were contributed for HCO3- efflux and activation of L-type calcium channels (31,71) and were blocked by bicuculline (competitive antagonist of GABAAR similar to picrotoxine, which is no competitive antagonist of GABAA receptor. **They have Cl- reversal similar o small HH neurons]
|
Large HH neurons |
Small HH neurons |
Cl- reversal potential |
-36.2±1.5 mV |
-59.4±3.9 mV |
Intracellular Cl- concentration |
39.1±2.8 mM |
19.2±3.7 mM |
Muscimol administration |
*Depolarizing 70% |
**Hyperpolarizing 30% |
Hyperpolarizing 100% |
| |
|
|
|
Reasons of modified Cl- reversal potential
- Altered expression of the cation-chloride transporters NKCC1 and KCC2 [28,34,46].
- Reduced expression of KCC2 mRNA in large HH neurons.
- Bumetanide (a NKCC1 inhibitor) partially blocks the depolarizing and firing behavior or large HH neurons exposed to GABA agonist [22,28]; so, there is an increasing of NKCC1 channels.
- Constitutive activation of tyrosine kinase β (trKβ), also known as tropomyosin kinase receptor complex down streaming intracellular signaling pathways (Figure 12).
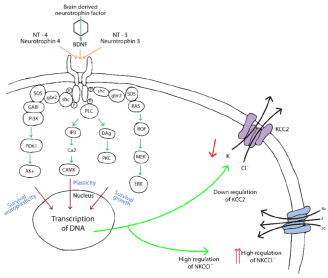
Figure 12. Scheme that depicts the tyrosin kinase B receptor (trkB) also named Tropomyosine kinase receptor, the stimulation of this kind of receptor by Neurotrophin-4 and 3 as well as Brain derived neurotrophin factor induces down regulation of KCC2, ending in diminishing potassium and chloride and increasing regulation of NKCCl, all these thanks to the transcription of DNA favored by second messengers induced by the complex trk-B and its agonists
Seizures Onset: Figure 13.
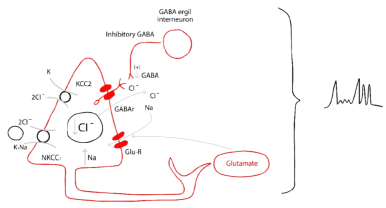
Figure 13. Intra and inter cellular pathophysiology of the seizures onset in HH
Suppression of seizures: Figure 14.
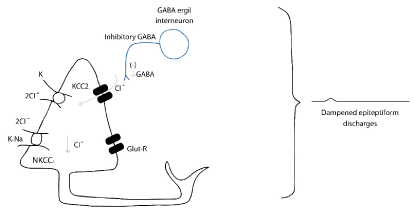
Figure 14. Mechanism of suppression seizures in HH
Mechanism responsible for increased synchrony
There are 2 basic cellular conditions required for every epileptic network:
- Imbalance of excitatory and inhibitory influences in the direction of net excitation.
- Hyper synchrony of neuronal firing [28,58]
The contribution of synchronized firing of inhibitory neurons within the reticular nucleus as a fundamental contributing factor to generalized spike-wave discharges in the thalami-cortical circuit is well recognized [28,59]. GABAA receptor mediated synchronization also contributes to:
- Seizure –onset mechanisms in focal epilepsy (including mesial temporal sclerosis [28,60,61] and cortical dysplasia [28,62].
- Post-synaptic evidence for pacemaker-like GABA-firing as a contributing factor for epileptic network synchronization in cortical dysplasia [28,63].
A possible contributing mechanism to GABA-mediated synchrony is the phenomenon of GABAA current rundown, which is determined experimentally by observing decreasing trans membrane current responses to repetitive (use-dependent) GABA-ligand exposure and has been related to: temporal lobe epilepsy [28,64,65] and cortical dysplasia [28,66]; furthermore, has been seen in surgically-resected HH tissue [28,67].
The functional rundown was specific to GABA (It was not observed in response to repetitive exposure to glycine or glutamate) and was also specific to GABAA receptors expressed on the surface of small HH neurons and not in large HH neurons [28,67].
Synchrony within small HH neuron clusters may also be enabled by non-synaptic mechanism [28,68]: neuronal gap junctions (connexin 36) are highly expressed within small HH neuron clusters, and microelectrode field recordings of seizure-like discharges in surgically resected HH tissue slices are significantly reduced by pharmacologic exposure to gap junction blockers (Figure 15).
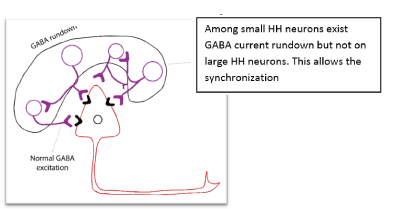
Figure 15. Mechanism of synchronization among small and large cells in HH
Secondary epileptogenesis beyond the HH
Since the 1990s, stereotactic intracerebral EEG and subsequent functional imaging studies in patients with epilepsy have demonstrated that HH is intrinsically epileptogenic [10,15,22]. However, multiple other seizure types were associated with ictal discharges affecting various neocortical areas but sparing the HH [10,15,17]. Collectively, these findings suggested the hypothesis of a secondary epileptogenesis as a possible underlying mechanism [10,15,17,69].
Despite the inefficiency of procedures aimed at destroying/ inhibiting/ disconnecting the HH in a non-negligible proportion of cases, “hamartoma-centrism” is generally the rule in the medical community.
Growing experience in epilepsy surgery addressing the HH showed that only (at best) 50% of the patients could be completely cured by the isolated treatment of the HH [15,70]. A partial improvement is obtained in others, with persistence of electro-clinical seizure patterns suggesting neocortical involvement [15,71].
Secondary epileptogenesis could be defined as the induction of epileptic activities in the cellular elements of a previously normal neural network by interconnected actively discharging epileptogenic area [15,72].
This process might be related to kindling [15,73] which refers to the tendency of the brain to become progressively more epileptogenic when stimulated repeatedly at an intensity that initially is under the threshold for the generation of epileptic potential.
Morrel postulated three stages of secondary epileptogenesis [15]:
- Stage 1: Dependent stage
Epileptogenic discharges detected in the secondary focus were driven by the primary focus [15,74,75].
- Stage 2: Intermediate stage
Independent inter ictal epileptiform discharges and later independent seizures were detected in the secondary focus. They initially persisted after removal of the primary focus, but eventually “ran down” and disappeared [15].
- Sage 3: Independent stage
Epileptiform activity in the secondary epileptogenic area persisted after removal of the primary focus without running down [15].
Secondary epileptogenesis has been studied extensively in animals over the last 50 years, yet its existence and role in human epilepsy remain a controversial issue [15,24,76]. Some authors consider epilepsy associated with HH as a model of secondary epileptogenesis, at least for the first two stages [15,70,77,78].
To date, there is no animal model for epilepsy with HH. Some common points could be discussed with the well-established model of audiogenic epilepsy in rodents [15,79]: during audiogenic kindling, epileptic discharge generated initially in the inferior colliculus, spreads from the brainstem to the forebrain and can progressively involve the hippocampus as well as widely distributed neocortical networks, finally leading to generalized tonic-clonic seizures [15,80].
However, the projection pathways from the subcortically situated primary epileptogenic area toward the limbic circuit are probably quite different from those supposed to be implied in epilepsy with HH.
Strictly speaking, hypothalamic-kindling phenomenon has been demonstrated in mice [15,81]. After-discharges could be induced in almost all regions of the hypothalamus, with a kindling rate similar to that of the amygdala, although the hypothalamus required a greater amount of stimulation before after-discharge threshold was reached.
In experimental animals, the time required to induce a secondary epileptic focus by kindling is known to increase across the species from amphibians to primates along with the rising complexity of the involved neural networks. Electrographic changes corresponding to a secondary focus occur within hours in the amphibian brain, within days to weeks in mice, within weeks to months in squirrel monkeys, and within months to years in macaques [15,76,82].
The location of the secondary focus would be depending on the cortical-cortical connections and can occur in homologous contralateral cortex (mirror focus) or in a different, but connected region [15,82].
The kindling has been postulated as a cause of secondary epileptogenesis in HH. To date, the direct clinical evidence for the independent stage of secondary epileptogenesis is lacking. However, it has been observed that additional seizure types often appeared with clear delay following the start of gelastic seizures during the clinical course [15,42,44,83].
In the Strasbourg-Kork series of 15 patients explored for an HH [15,84] dyscognitive seizures with electro-clinical features suggesting that temporal, frontal or parietal lobe involvement were documented in all but one case; however maturational changes may also explain the development of independent seizures [15].
The connectivity of hypothalamus with the ipsilateral thalamus, mainly with anterior nucleus (being relay to the anterior cingulate and limbic circuit) and dorso-medial thalamus (relay to orbitofrontal cortex, mesial premotor cortex, anterior cingulate) and the default mode network (precuneus and lateral inferior parietal lobe) [1], have been shown in ictal single photon emission computed tomography (SPECT) studies [11,15,79,85].
Inter ictal hypometabolism of ipsilateral thalamus was demonstrated by 18 FDG-PET [15,86].
Scalp and invasive recordings demonstrated an almost exclusive lateralization of neocortical interictal epileptiform discharges (IEDs) and intracranially recorded seizures ipsilaterally to the site of HH [15,17].
The ultimate “proof of concept” for intrinsic epileptogenesis of HH is that its complete surgical resection (or treatment with other tissue-destructive therapeutic modalities) stops gelastic seizures [15,84]. Other seizure types can also disappear following HH removal, whereas isolated neocortical resection generally fail to control seizures [15,69,87]. Some studies have demonstrated that complete recovery from neocortical seizures could be delayed, occurring up to 6 months after HH ablation [39,15]. These data argue for the running-down phenomenon suggesting a reversal of secondary epileptogenesis at its second, intermediate stage as described by Morell [15,72].
A relationship between epilepsy duration and surgical outcome has been observed in some series [15,31,88]: shorter duration of epilepsy seemed to be associated with better outcomes in the Strasbourg-Korj series:
- Less than 10 years of epilepsy before surgery à 80% achieved seizure free
- Between 11-20 years before surgery à 60% achieved seizure free
- More than 20 years before surgery à 20% achieved seizure free
According to the experience obtained by SEEG (stereotactic EEG), Munari et al. [10,15] reported a detailed stereotactic exploration of a patient presenting with gelastic seizures and partial seizures suggestive of frontal involvement: gelastic seizures were linked to rapid discharges in the HH, whereas tonic motor seizures were associated with a discharge extended to the hypothalamus and the cortical motor regions.
Mahone et al. [15,17] reported the complete Grenoble series (five cases) where gelastic or dacrystic seizures were correlated with discharges in the HH, but other kinds of seizures were related to discharges affecting the neocortical regions.
They proposed that a large part of this kindling was related to the involvement of the cingulate gyrus [15,79].
In Grenoble’s experience, SEEG-guided radiofrequency thermos-coagulations were performed through the contacts located in the HH and led to a decrease of gelastic but not of tonic seizures.
Diagnosis in HH
The mechanisms for getting the suitable diagnostic work is (Table 16):
Table 16. Role of the Glucagon stimulation testing
*Glucagon stimulation testing [30]
- The α-cell of the Langerhans Island in the pancreas normally produces glucagon. It induces the gluconeogenesis by reduction of fructose 2,6 diphosphate. Glucagon increases the level of glucose leading to augmentation of insulin levels, the latter carry to decreasing of the level of glucagon
- This test h2021 Copyright OAT. All rights reserv hormone deficiency (GHD).
- It is not well known how glucagon induces ACTH and thus increases cortisol levels.
- Glucagon (0.1 mg/kg), only by intramuscular or subcutaneous application (not intravenous), induces release of GH, ACTH. Seeming this is caused by glucagon activity directly on the hypophysis and increasing activity on α receptors.
- It is also used for evaluating pancreatic reserve (insulin reserve): deficit of GH, reserve of ACTH.
|
- Regarding the epilepsy symptoms:
- Routine scalp EEG monitoring
- Long term video EEG monitoring
- Ictal and interictal SPECT
- MRI
- Neuropsychological evaluation
- Ophthalmological assessment and perimetry
- About the endocrine assessment
- Sex hormone
- Growth hormone levels
- Thyroid function
- Prolactin level
- Cortisol reserve with glucagon stimulation*
Surgery
Surgical resection for total removal of HHs has led to good seizure outcomes and behavioral improvement [1,89-91]
The different options of treatment for HH found in the literature are:
- Surgery: “from above”: trans-callosal, inter-fornicial approach (more dangerous for the hypothalamus and fornix)
- Endoscopic surgery: disconnection technique
- Gamma knife surgery
- Stereotactic radiofrequency thermos coagulation: disconnection technique
According to the Delalande and Fohlen classification, it is possible to recommend a specific therapeutic strategy depends on the localization of the tumor: Figure 16.
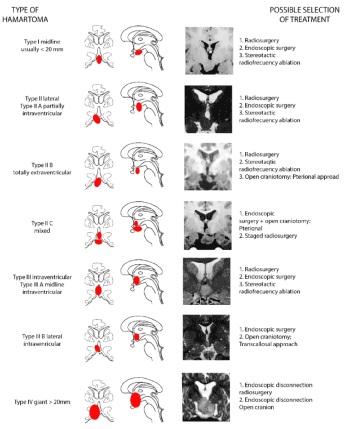
Figure 16. Classification of the HH according to the volume and the implantation. A large Hamartoma (>20 mm) is defined as a giant HH (Type IV). Small HHs (<20 mm) are classified as midline Type I and lateral Type II and intraventricular (Type III) according to their location relative to the third ventricle. Taken from Delalande O, et al. [28]
Endoscopic procedure for HH
For the endoscopic surgical treatment of HH a 3.8 mm outside diameter neuroendoscope is advisable. The procedure is performed through Kocher´s point.
Once the telescope has been advanced through the foramen of Monroe, the HH protruding from the floor and the lateral wall of the third ventricle is seen; it is easily defined the place where the HH is attached, however for difficult cases, the depth electrode inserted prior to endoscope surgery to guide the depth and the margin of disconnection is useful.
The disconnection will begin at the border between the HH and the mammillary body and proceed along the midline postero-inferior floor of the third ventricle. The direction of resection is from posterior to anterior and from lateral to inferior midline.
Neuronavigation endoscopy may be a very useful help to guide the resection.
Discussion
Usually, patients with HH suffered of refractory epilepsy and the frequency of seizures / month are high. Few cases debuted with Gelastic seizures (14%), nonetheless, with the maturation of the epileptogenic zone as well as the epileptic networks, the patients were increasing the number of GS with the time (being the 42% of the seizures before surgery). Other kind of seizures seen in HH are focal motor, focal temporal and generalized.
Commonly the patients with HH have other neurological symptoms such as behavioral disorders, endocrinological disturbances and visual impairment.
Mental retardation is common in patients older than 5 years in HH, there is also a delay in neurological development in younger ages.
In our experience most of the HH had wide implantation inside the third ventricle. We did not find difference in the frequency of seizures or electrophysiological findings respect to the side.
Only 14% of the patients had a normal preoperative baseline EEG, and most had a slow rhythm with theta waves, lateralizing to the same side of the lesion; commonly ictal findings were: spikes, poli spikes and spike- slow wave.
The EEG was able to lateralize correctly in 86% of the cases
All patients were operated with open surgery using trans-callosal or trans-silvian approach, indistinctly of the technique only one patient had a total resection.
The most common complication was the third nerve palsy. The stay in ICU was short (mode of 2 days).
86% of the patients after surgery experienced improvement in the frequency of seizures (Engel I, II and III) from this proportion 42.85% were Engel I; only 14% of the patients worsened. (Engel IV).
The size of the HH was not an important factor in the result (one patient with large volume of the lesion resulted with Engel Ia and the another one with similar volume had an outcome defined as IIIc). This finding requires to be analyzed deeper in future studies.
The patient’s epilepsy was partially controlled but was not possible to reduce the AEDs; probably because the partial resection performed, allowed some kind of disconnection. Notwithstanding of great resection performed or the type of approach, we did not find a deterioration in mental development after surgery.
Conclusions
The pre-surgical condition of the patients has the same features respect to the description in the global literature. It was interesting that only 14% of our patients presented with gelastic seizures. However, with the time, near to 42% had them, possibly secondary to the maturation of the epileptogenic zone and network. The experience in our institution about HH and epilepsy refractory to medical treatment is particularly using open surgery. In spite of the open technique used, the possibility to remove the totality of the tumor was only 14%. Nonetheless the results according to the international parameters (ILAE’s and Engel’s outcome score) for evaluating the successful is acceptable, possibly because the surgery finishes disconnecting the hamartoma from the rest of the hypothalamus. The 84% are classified with improvement after surgery and 42.85% are currently without seizures. We did not have behavioral complications or changes in the neuropsychological evaluation after surgery. Respect to the impact of the surgery on the number of medications or doses used of AEDs, only 29% was possible to reduce them, no one could discontinue the medication, even 57% had to increase the AEDs. We believe the patients became with the surgery “AEDs sensitive”, possibly due to the mass reduction. We hypothesized that partial removal of the tumor reduces the charge of the smallest neurons implicated in the synchronization on the large neurons. It should be noted that patients in whom seizures did not subside with the surgical treatment were treated with benzodiazepines, drugs that act at the level of chlorine channels
Conflict of interest
None.
References
- Shim KW, Park EK, Kim DS (2017) Endoscopic Treatment of Hypothalamic Hamartomas.J Korean Neurosurg Soc60: 294-300. [Crossref]
- Arroyo S, Santamaria J, Sanmarti F, Lomena F, Catafau A, et al. (1997) Ictal laughter associated with paroxysmal hypothalamopituitary dysfunction. Epilepsia 38: 114-117. [Crossref]
- Cerullo A, Tinuper P, Provini F, Contin M, Rosati A, et al. (1998) Autonomic and hormonal ictal changes in gelastic seizures from hypothalamic hamartomas. Electroencephalogr Clin Neurophysiol 107: 317- 322. [Crossref]
- Boyko OB, Curnes JT, Oakes WJ, Burger PC (1991) Hamartomas of the tuber cinereum: CT, MR, and pathologic findings.AJNR Am J Neuroradiol12: 309-314. [Crossref]
- Arita K, Ikawa F, Kurisu K, Sumida M, Harada K, et al. (1999) The relationship between magnetic resonance imaging findings and clinical manifestations of hypothalamic hamartoma. J Neurosurg 91: 212-220. [Crossref]
- Gascon GG, Lombroso CT (1971) Epileptic (gelastic) laughter.Epilepsia12: 63-76. [Crossref]
- Gumpert J, Hansotia P, Upton A (1970) Gelastic epilepsy.J Neurol Neurosurg Psychiatry33: 479-483. [Crossref]
- Berkovic SF, Arzimanoglou A, Kuzniecky R, Harvey AS, Palmini A, et al. (2003) Hypothalamic hamartoma and seizures: a treatable epileptic encephalopathy. Epilepsia 44: 969-973. [Crossref]
- Likavec AM, Dickerman RD, Heiss JD, Liow K (2000) Retrospective analysis of surgical treatment outcomes for gelastic seizures: a review of the literature. Seizure 9: 204-207. [Crossref]
- Munari C, Kahane P, Francione S, Hoffmann D, Tassi L, et al. (1995) Role of the hypothalamic hamartoma in the genesis of gelastic fits (a video-stereo-EEG study).Electroencephalogr Clin Neurophysiol95: 154-160. [Crossref]
- Kameyama S, Masuda H, Murakami H (2010) Ictogenesis and symptomatogenesis of gelastic seizures in hypothalamic hamartomas: an ictal SPECT study.Epilepsia51: 2270-2279. [Crossref]
- Khan S, Wright I, Javed S, Sharples P, Jardine P, et al. (2009) High frequency stimulation of the mamillothalamic tract for the treatment of resistant seizures associated with hypothalamic hamartoma. Epilepsia 50: 1608-1611. [Crossref]
- Usami K, Matsumoto R, Sawamoto N, Murakami H, Inouchi M, et al. (2016) Epileptic network of hypothalamic hamartoma: An EEG-fMRI study.Epilepsy Res125: 1-9. [Crossref]
- Troester M, Haine-Schlagel R, Ng YT, Chapman K, Chung S, et al. (2011) EEG and video-EEG seizure monitoring has limited utility in patients with hypothalamic hamartoma and epilepsy. Epilepsia 52: 1137-1143. [Crossref]
- Scholly J, Staack AM, Kahane P, Scavarda D, Régis J, et al. (2017) Hypothalamic hamartoma: Epileptogenesis beyond the lesion?Epilepsia58 Suppl 2: 32-40. [Crossref]
- DiFazio MP, Davis RG (2000) Utility of early single photon emission computed tomography (SPECT) in neonatal gelastic epilepsy associated with hypothalamic hamartoma. J Child Neurol 15: 414-417. [Crossref]
- Kahane P, Ryvlin P, Hoffmann D, Minotti L, Benabid AL (2003) From hypothalamic hamartoma to cortex: what can be learnt from depth recordings and stimulation?Epileptic Disord5: 205-217. [Crossref]
- Shim KW, Chang JH, Park YG, Kim HD, Choi JU, et al. (2008) Treatment modality for intractable epilepsy in hypothalamic hamartomatous lesions. Neurosurgery 62: 847-856. [Crossref]
- Specchio N, Rizzi M, Trivisano M, Fusco L, Rebessi E, et al. (2015) Acute intralesional recording in hypothalamic hamartoma: description of 4 cases. Acta Neurol Belg 115: 233-239. [Crossref]
- Beggs J, Nakada S, Fenoglio K, Wu J, Coons S, et al. (2008) Hypothalamic hamartomas associated with epilepsy: ultrastructural features. J Neuropathol Exp Neurol 67: 657-668. [Crossref]
- Coons SW, Rekate HL, Prenger EC, Wang N, Drees C, et al. (2007) The histopathology of hypothalamic hamartomas: study of 57 cases.J Neuropathol Exp Neurol66: 131-141. [Crossref]
- Kim do Y, Fenoglio KA, Simeone TA, Coons SW, Wu J, et al. (2008) GABAA receptor-mediated activation of L-type calcium channels induces neuronal excitation in surgically resected human hypothalamic hamartomas. Epilepsia 49: 861-871. [Crossref]
- Wu J, Xu L, Kim DY, Rho JM, St John PA, et al. (2005) Electrophysiological properties of human hypothalamic hamartomas. Ann Neurol 58: 371-382. [Crossref]
- Steinmetz PN, Wait SD, Lekovic GP, Rekate HL, Kerrigan JF (2013) Firing behavior and network activity of single neurons in human epileptic hypothalamic hamartoma. Front Neurol 4: 210. [Crossref]
- Roberts CM, Thompson EM, Selden NR (2011) Transendoscopic intraoperative recording of gelastic seizures from a hypothalamic hamartoma. Pediatr Neurosurg 47: 147-151. [Crossref]
- Striano S, Striano P, Cirillo S, Nocerino C, Bilo L, et al. (2002) Small hypothalamic hamartomas and gelastic seizures. Epileptic Disord 4: 129-133. [Crossref]
- Valdueza JM, Cristante L, Dammann O, Bentele K, Vortmeyer A, et al. (1994) Hypothalamic hamartomas: with special reference to gelastic epilepsy and surgery. Neurosurgery 34: 949-958. [Crossref]
- Wu J, Gao M, Shen JX, Qiu S, Kerrigan J (2015) Mechanisms of intrinsic epileptogenesis in human gelastic seizures with hypothalamic hamartoma. CNS Neurosci Ther 21: 104-111. [Crossref]
- Berkovic SF, Andermann F, Melanson D, Ethier RE, Feindel W, et al. (1988) Hypothalamic hamartomas and ictal laughter: Evolution of a characteristic epileptic syndrome and diagnostic value of magnetic resonance imaging. Ann Neurol 23: 429–439.
- Choi JU, Kim DS (2012) Treatment modalities for intractable epilepsy in hypothalamic hamartoma.Adv Tech Stand Neurosurg39: 117-130. [Crossref]
- Ng YT, Rekate HL, Prenger EC, Chung SS, Feiz-Erfan I, et al. (2006) Transcallosal resection of hypothalamic hamartoma for intractable epilepsy. Epilepsia 47: 1192–1202. [Crossref]
- Berkovic SF, Kuzniecky RI, Andermann F (1997) Human epileptogenesis and hypothalamic hamartomas: New lessons from an experiment of nature. Epilepsia 38: 1–3.
- Striano S, Meo R, Bilo L, Cirillo S, Nocerino C, et al. (1999) Gelastic epilepsy: symptomatic and cryptogenic cases.Epilepsia40: 294-302. [Crossref]
- Kuzniecky R, Guthrie B, Mountz J, Bebin M, Faught E, et al. (1997) Intrinsic epileptogenesis of hypothalamic hamartomas in gelastic epilepsy.Ann Neurol42: 60-67. [Crossref]
- Wu J, Chang Y, Li G, Xue F, DeChon J et al. (2007) Electrophysiological properties and subunit composition of GABAA receptors in patients with gelastic seizures and hypothalamic hamartoma. J Neurophysiol 98: 5–15. [Crossref]
- Freeman JL, Harvey AS, Rosenfeld JV, Wrennall JA, Bailey CA, et al. (2003) Generalized epilepsy in hypothalamic hamartoma: Evolution and postoperative resolution. Neurology 60: 762–767. [Crossref]
- Castro LH, Ferreira LK, Teles LR, Jorge CL, Arantes PR, et al. (2007) Epilepsy syndromes associated with hypothalamic hamartoma. Seizure 16: 50–58. [Crossref]
- Parvizi J, Le S, Foster B, Bourgeois B, Riviello JJ, et al. (2011) Gelastic epilepsy and hypothalamic hamartomas: Neuroanatomical analysis of brain lesions in 100 patients. Brain 134: 2960–2968. [Crossref]
- Sturm JW, Andermann F, Berkovic SF (2000) “Pressure to laugh”: An unusual epileptic syndrome associated with small hypothalamic hamartomas. Neurology 54: 971–973. [Crossref]
- Striano S, Striano P, Sarappa C, Boccella P (2005) The clinical spectrum and natural history of gelastic epilepsy-hypothalamic hamartoma syndrome. Seizure 14: 232–239. [Crossref]
- Sato M, Ushio Y, Arita N, Mogami H (1985) Hypothalamic hamartoma: report of two cases.Neurosurgery16: 198-206. [Crossref]
- Palmini A, Chandler C, Andermann F, Costa Da Costa J, Paglioli-Neto E, et al. (2002) Resection of the lesion in patients with hypothalamic hamartomas and catastrophic epilepsy.Neurology58: 1338-1347. [Crossref]
- Cepeda C, Andre VM, Levine MS, Salamon N, Miyata H, et al. (2006) Epileptogenesis in pediatric cortical dysplasia: The dysmature cerebral developmental hypothesis. Epilepsy Behav 9: 219–235. [Crossref]
- Cepeda C, André VM, Wu N, Yamazaki I, Uzgil B, et al. (2007) Immature neurons and GABA networks may contribute to epileptogenesis in pediatric cortical dysplasia.Epilepsia48 Suppl 5: 79-85. [Crossref]
- Wu J, DeChon J, Xue F, Li G, Ellsworth K, et al. (2008) GABAA receptor-mediated excitation in dissociated neurons from human hypothalamic hamartomas. Exp Neurol 213: 397–404. [Crossref]
- Macdonald RL, Olsen RW (1994) GABAA receptor channels.Annu Rev Neurosci17: 569-602. [Crossref]
- Farrant M, Nusser Z (2005) Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors.Nat Rev Neurosci6: 215-229. [Crossref]
- Benarroch EE (2007) GABAA receptor heterogeneity, function, and implications for epilepsy.Neurology68: 612-614. [Crossref]
- Ben-Ari Y (2002) Excitatory actions of gaba during development: the nature of the nurture.Nat Rev Neurosci3: 728-739. [Crossref]
- Stein V, Nicoll RA (2003) GABA generates excitement.Neuron37: 375-378. [Crossref]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL (1989) Giant synaptic potentials in immature rat CA3 hippocampal neurones.J Physiol416: 303-325. [Crossref]
- Kim DY, Fenoglio KA, Kerrigan JF, Rho JM (2009) Bicarbonate contributes to GABAA receptor-mediated neuronal excitation in surgically resected human hypothalamic hamartomas. Epilepsy Res 83: 89–93. [Crossref]
- Moenter SM, DeFazio RA (2005) Endogenous gamma-aminobutyric acid can excite gonadotropin-releasing hormone neurons. Endocrinology 146: 5374–5379. [Crossref]
- Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R (2002) On the origin of interictal activity in human temporal lobe epilepsy in vitro.Science298: 1418-1421. [Crossref]
- Cossart R, Bernard C, Ben-Ari Y (2005) Multiple facets of GABAergic neurons and synapses: Multiple fates of GABA signalling in epilepsies. Trends Neurosci 28: 108–115. [Crossref]
- Khalilov I, Holmes GL, Ben-Ari Y (2003) In vitro formation of a secondary epileptogenic mirror focus by interhippocampal propagation of seizures. Nat Neurosci 6: 1079–1085. [Crossref]
- Andermann F, Arzimanoglou A, Berkovic SF (2003) Hypothalamic hamartoma and epilepsy: the pathway of discovery.Epileptic Disord5: 173-175. [Crossref]
- Prince DA (1985) Physiological mechanisms of focal epileptogenesis.Epilepsia26 Suppl 1: S3-14. [Crossref]
- Huguenard JR, McCormick DA (2007) Thalamic synchrony and dynamic regulation of global forebrain oscillations. Trends Neurosci 30: 350–356. [Crossref]
- Kohling R, Vreugdenhil M, Bracci E, Jeffreys JGR (2000) Ictal epileptiform activity is facilitated by hippocampal GABAA receptor-mediated oscillations. J Neurosci 20: 6820–6829. [Crossref]
- Behr C, D'Antuono M, Hamidi S, Herrington R, Lévesque M, et al. (2014) Limbic networks and epileptiform synchronization: the view from the experimental side.Int Rev Neurobiol114: 63-87. [Crossref]
- D’Antuono M, Louvel J, Kohling R, et al. (2004) GABAA receptormediated synchronization leads to ictogenesis in the human dysplastic cortex. Brain 127: 1626–1640. [Crossref]
- Cepeda C, Chen JY, Wu JY, Fisher RS, Vinters HV, et al. (2014) Pacemaker GABA synaptic activity may contribute to network synchronization in pediatric cortical dysplasia. Neurobiol Dis 62: 208–217. [Crossref]
- Palma E, Roseti C, Maiolino F, Fucile S, Martinello K, et al. (2007) GABA(A)-current rundown of temporal lobe epilepsy is associated with repetitive activation of GABA(A) "phasic" receptors.Proc Natl Acad Sci U S A104: 20944-20948. [Crossref]
- Cifelli P, Palma E, Roseti C, Verlengia G, Simonato M (2013) Changes in the sensitivity of GABAA current rundown to drug treatments in a model of temporal lobe epilepsy.Front Cell Neurosci7: 108. [Crossref]
- Jansen LA, Peugh LD, Ojemann JG (2008) GABAA receptor properties in catastrophic infantile epilepsy. Epilepsy Res 81: 188–197. [Crossref]
- Li G, Yang K, Zheng C, Liu Q, Chang Y, et al. (2011) Functional rundown of gamma-aminobutyric acid(A) receptors in human hypothalamic hamartomas. Ann Neurol 69: 664–672. [Crossref]
- Dudek FE, Yasumura T, Rash JE (1998) 'Non-synaptic' mechanisms in seizures and epileptogenesis.Cell Biol Int22: 793-805. [Crossref]
- Kerrigan JF, Ng YT, Chung S, Rekate HL (2005) The hypothalamic hamartoma: a model of subcortical epileptogenesis and encephalopathy. Semin Pediatr Neurol 12: 119–131. [Crossref]
- Kerrigan JF (2011) Hypothalamic hamartoma and gelastic epilepsy. In Shorvon SD, Andermann F, Guerrini R (Eds) The causes of Epilepsy. Cambridge: Cambridge University Press, pp: 449–453.
- Régis J, Carron R, Bartolomei F, Chauvel P (2012) Seeking new paradigms in epilepsy: stereotactic radiosurgery.Clin Neurosurg59: 59-69. [Crossref]
- Morrell F (1985) Secondary epileptogenesis in man.Arch Neurol42: 318-335. [Crossref]
- Goddard GV (1967) Development of epileptic seizures through brain stimulation at low intensity.Nature214: 1020-1021. [Crossref]
- Morrell F, Roberts L, Jasper HH (1956) Effect of focal epileptogenic lesions and their ablation upon conditioned electrical responses of the brain in the monkey. Electroencephalogr Clin Neurophysiol 8: 217–236. [Crossref]
- Morrell F, deToledo-Morrell L (1999) From mirror focus to secondary epileptogenesis in man: an historical review.Adv Neurol81: 11-23. [Crossref]
- Wilder BJ, Morrell F (1967) Secondary epileptogenesis in the frog forebrain.Neurology17: 1041-1051. [Crossref]
- Norden AD, Blumenfeld H (2002) The role of subcortical structures in human epilepsy.Epilepsy Behav3: 219-231. [Crossref]
- Valentin A, Lazaro M, Mullatti N, Cervantes S, Malik I, et al. (2011) Cingulate epileptogenesis in hypothalamic hamartoma.Epilepsia52: e35-39. [Crossref]
- Marescaux C, Vergnes M, Kiesmann M, Depaulis A, Micheletti G, et al. (1987) Kindling of audiogenic seizures in Wistar rats: an EEG study.Exp Neurol97: 160-168. [Crossref]
- Hirsch E, Maton B, Vergnes M, Depaulis A, Marescaux C (1992) Positive transfer of audiogenic kindling to electrical hippocampal kindling in rats.Epilepsy Res11: 159-166. [Crossref]
- Cullen N, Goddard GV (1975) Kindling in the hypothalamus and transfer to the ipsilateral amygdala.Behav Biol15: 119-131. [Crossref]
- Cibula JE, Gilmore RL (1997) Secondary epileptogenesis in humans.J Clin Neurophysiol14: 111-127. [Crossref]
- Striano S, Santulli L, Ianniciello M, Ferretti M, Romanelli P, et al. (2012) The gelastic seizureshypothalamic hamartoma syndrome: facts, hypotheses, and perspectives. Epilepsy Behav 24: 7–13. [Crossref]
- Scholly J, Valenti MP, Staack AM, Strobl K, Bast T, et al. (2013) Hypothalamic hamartoma: is the epileptogenic zone always hypothalamic? Arguments for independent (third stage) secondary epileptogenesis. Epilepsia 54: 123–128. [Crossref]
- Arroyo S, Lesser RP, Gordon B, Uematsu S, Hart J, et al. (1993) Mirth, laughter and gelastic seizures.Brain116: 757-780. [Crossref]
- Ryvlin P, Ravier C, Bouvard S, Mauguire F, Le Bars D, et al. (2003) Positron emission tomography in epileptogenic hypothalamic hamartomas. Epileptic Disord 5: 219–227. [Crossref]
- Cascino GD, Andermann F, Berkovic SF, Kuzniecky RI, Sharbrough FW, et al. (1993) Gelastic seizures and hypothalamic hamartomas: evaluation of patients undergoing chronic intracranial EEG monitoring and outcome of surgical treatment. Neurology 43: 747–750. [Crossref]
- Schulze-Bonhage A1, Trippel M, Wagner K, Bast T, Deimling FV, et al. (2008) Outcome and predictors of interstitial radiosurgery in the treatment of gelastic epilepsy.Neurology71: 277-282. [Crossref]
- Delalande O, Fohlen M (2003) Disconnecting surgical treatment of hypothalamic hamartoma in children and adults with refractory epilepsy and proposal of a new classification. Neurol Med Chir (Tokyo) 43: 61-68. [Crossref]
- Rosenfeld JV, Freeman JL, Harvey AS (2004) Operative technique: the anterior transcallosal transseptal interforniceal approach to the third ventricle and resection of hypothalamic hamartomas. J Clin Neurosci 11: 738-744. [Crossref]
- Yuen KC (2011) Glucagon stimulation testing in assessing for adult growth hormone deficiency: current stats and future perspectives. ISRN Endocrinol 2011: 608056. [Crossref]
















