Background and Objectives: BK virus nephropathy (BKVN) occurs in kidney transplant recipients as a result of BK virus (BKV) reactivation and can often result in graft loss. Reconstitution of cell mediated immune response (CMIR) has been shown to correlate with resolution of nephropathy. We aim to show that there is a delay between immune reconstitution and viral clearance and that immune monitoring in conjunction with BK virus qPCR is a better tool than qPCR alone to guide treatment recommendations for BKVN.
Materials and Methods: This is a prospective observational study of 4 living donor renal transplant recipients with BK Viremia with Cylex ImmuKnowTMTest (Cylex Inc., Columbia, MD) in conjunction with BKV qPCR monitoring until viral clearance. All patients received thymoglobulin induction and tacrolimus, mycophenolate mofetil, and steroids for maintenance. We collected data on CMIR with cylex testing, BKV qPCR, S. Cr, renal biopsy, and rate of acute rejection following end of treatment.
Results: We found an inverse correlation between cylex levels and BK viremia. All patients with BKVN were managed with immunosupression reduction and antiviral therapy, based on our institutional protocol. When the BKV titer was <3000 copies and cylex was >200, we restarted MMF or resumed its normal dose. Leflunomide was the antiviral used and it was stopped as soon as BKV titer was less than 10,000 copies/mL.
Conclusions: CMIR monitoring using the readily available Cylex Test with BKV quantitative polymerase chain reaction (qPCR) enabled us to personalize BKV treatment and prevent rejection episodes.
BK Virus nephropathy, kidney transplant, immune monitoring, cylex, cell mediated immune response
BKV: BK virus; BKVN: BK virus nephropathy; CMIR: cell mediated immune response; MMF: mycophenolate mofetil; JCV: JC virus; IFNγ: interferon-gamma; qPCR: quantitative polymerase chain reaction; ATP: adenosine triphosphate; ELISPOT: Enzyme-Linked ImmunoSpot; IS: Immunnosuppression; PML: Progressive Multifocal Leukoencephalopathy.
BK virus (BKV) was isolated from the urine sample of a renal transplant patient in 1971, and named after the patients' initials [1]. BKV is a non-enveloped virus with a genome of double stranded circular DNA. Primary BKV infection is acquired during childhood, most likely from person to person contact from respiratory secretions; and usually manifests as mild respiratory infection or fever [2]. The virus multiplies in respiratory tract, and then spreads to other organs though blood stream. BKV remains clinically silent in immunocompetent individuals.
BKV reactivation is observed following transplantation due to immunosuppression (IS). Clinically, BKV reactivation begins with active viral replication in the graft, followed by viral shedding in the urine, which can be seen in 20-60% patients, and finally, viremia. The incidence of BK viremia in kidney transplant recipients is about 13%, while BK virus nephropathy (BKVN, defined by BKV quantitative polymerase chain reaction (qPCR) in blood >10,000 copies/mL for >1 month) is seen in nearly 8%, which can often result in graft loss [3]. Multicenter national data from United Network for Organ Sharing (UNOS) shows that graft loss attributable to BKVN was 7.5% in 2009 and 5.7% in 2010 [4]. BKVN is therefore, a serious clinical problem in kidney transplantation.
While most of the population is seropositive for BKV antibodies, it is likely that humoral immune response alone does not play a significant role in preventing progression to BKVN, since patients with levels of anti-BKV antibodies similar to those of healthy individuals still develop BKVN [5]. High levels of antibodies in patients with BKVN correlate with high levels of viremia and low CD8+ T cell responses [6]. BKV-specific cell mediated immune response (CMIR) was demonstrated in normal individuals to be the mechanism important in preventing viral reactivation in healthy volunteers [7]. This is further supported by the study of cellular immune response to another related virus, JC virus (JCV), in patients with Progressive Multifocal Leukoencephalopathy (PML), where it was shown that JCV specific cytotoxic T lymphocytes were a key factor in containment of PML [8]. Low levels of BKV specific interferon-gamma (IFNγ) producing T cells correlate with progression to BKVN, while reconstitution of these cells correlates with resolution of nephropathy [9-12]. In this pilot study, we propose to evaluate Immune monitoring with cylex along with qPCR as a better marker for guiding anti-BKV treatment strategy compared to qPCR alone.
This was a prospective observational study of immune monitoring for BK virus in kidney transplant recipients over a 1-year period, following IRB approval (HSIRB #528500). Consecutive adult (>18 year old) kidney transplant recipients receiving their first transplant with BKVN regardless of gender, age, or race; were included in the study, while kidney transplant recipients who were highly sensitized (high PRA, previous transplant); patients with cancer, HIV, Hepatitis B or Hepatitis C, adults unable to consent, pregnant women, and prisoners, were excluded from the study. All recipients underwent monthly screening post-transplant for BK virus DNA in blood using qPCR, and Cylex testing. All patients with BK viremia were treated as per standard institutional protocol (Figure 1).
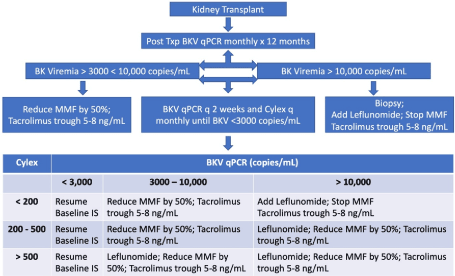
Figure 1. BKV treatment protocol at our center
We collected data on CMIR with cylex testing, BKV qPCR, S. Cr, renal biopsy, and rate of acute rejection following end of treatment. We collected Cylex measurements at 1-month intervals during viremia, and BKV qPCR two weekly during viremia and monthly thereafter, along with serum creatinine (Cr) as described in Table 1.
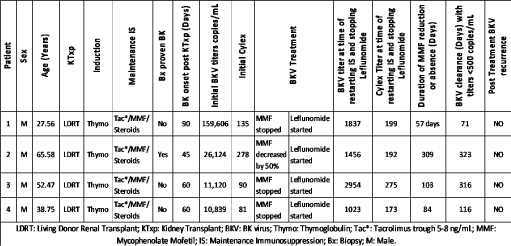
Table 1. Patient Data
Due to a small number of patients in this pilot study to obtain proof of concept, we present the data as a descriptive analysis (Table 1). There were four male recipients of living donor kidney transplants aged, 27.56, 38.75, 52.47, and 65.58 years. All received induction with Thymoglobulin 4.5 mg/Kg in three divided doses, and maintenance IS with tacrolimus to target trough levels of 8-10 ng/mL, MMF, 1 gm PO BID, and prednisone taper to 5 mg PO daily by 6 weeks post-transplant. BK Viremia onset ranged from 45 – 90 days post-transplant, and initial BK titers ranged from 10,839 to 159,606 copies/mL, while initial cylex levels ranged from 81 – 278 indicating that all patients were quite immunosuppressed. All were treated per institutional protocol described in Figure 1. At treatment endpoint when IS was resumed at baseline, the BK titers ranged from 1023 to 2954 copies/mL, with mean value of 1817.5 copies/mL, while cylex titers ranged from 173 to 275 with mean of 209.75. The time required to achieve BK titers of <3,000 copies/mL, which was defined as treatment endpoint based on our institutional experience, ranged from 84 to 309 days following onset of treatment. Baseline IS was restarted at this point. However, time to achieve BK virus clearance from blood defined as BK titers < 500 copies/mL ranged from 71 to 323 days. For patients 1 and 2, it was 14 days following treatment endpoint, for patient 3 it was 213 days, and for patient 4, it was 32 days following treatment endpoint. This highlights the importance of restarting IS at BKV titers < 3000 copies/mL (based on upper limit of range in our study) without waiting for viral clearance in blood, since immune reconstitution, defined by cylex levels of > 200 (based on upper limit of range) in our study, occurred much before viral clearance. If we had not resumed IS at that point, some of these patients might have had a rejection episode. Leflunomide was stopped for BKV level was less than 10,000 copies/mL. No adverse events were reported in any patient with leflunomide.
BKVN is very difficult to treat since there is no BKV-specific anti-viral therapy. Any anti-virals currently in use, work poorly, and suffer from substantial host toxicity. BKVN usually implies excessive immunosuppression and is treated by stimulating host immune response by IS reduction. However, acute rejection is frequently observed following virus clearance [13], further complicating treatment options since rejection treatment requires escalation of IS which could result in BKV recurrence, and graft dysfunction. The most effective strategy for early diagnosis and treatment is regular monitoring for BKV, which is currently achieved by quantification of viral DNA in blood by qPCR. Although it is widely known that reconstitution of CMIR plays a crucial role in recovery from BKVN, this knowledge has not been used in BKV treatment [14]. We demonstrated in this pilot study that there is a delay between immune reconstitution and viral clearance thereby making a case for CMIR monitoring for treating all patients with BKVN to establish new treatment guidelines.
Therefore, there is a need to evaluate alternative markers for determination of treatment endpoint for BKVN. One such alternative is measurement of cell-mediated immune response using a previously validated T cell function assay ((ImmuKnowTM; Cylex Inc. Columbia, MD), which measures immune cell function based on the amount of adenosine triphosphate (ATP) released when CD4+ T cells are stimulated by phytohemagglutinin. It has been previously validated in kidney transplant recipients with BKVN, and in a study demonstrated that a decreased test result correlates with active viral replication in kidney transplant recipients [9]. This test is readily available commercially. An alternative approach to measure BKV-specific CMIR could be to use Enzyme-Linked ImmunoSpot (ELISPOT) assay to quantify the concentration of T cells in patient’s PBMC that produce IFNγ in response to activation by a broad panel of BK peptide epitopes as described by Schachtner, et al. [15]. However, the limitation of ELISPOT assay is that it cannot confirm the identity of the IFNγ producing cells as activated T cells using concurrent CD4 and CD8 staining. CD4 or CD8 cells can separated by magnetic bead separation or FACS cell sorting, but it would then be necessary to include purified antigen presenting cells with the antigen in the ELISPOT analysis. Due to lack of funding we were not able to use ELISPOT or FACS cell sorting; so, in order to get a proof of concept we decided to use Cylex testing, which we were already using in our center to guide treatment for rejections, BK viremia and CMV viremia.
Our choice of antiviral was Leflunomide. It is an anti-inflammatory agent approved for use in rheumatoid arthritis. It has unique antiviral and immunosuppressive properties, and is well tolerated, making it useful in treatment of BKV nephropathy [16-18]. It is administered as a loading dose of 100 mg daily for 3 to 5 days followed by a maintenance dose of 20 to 60 mg daily with target trough levels of 50 to 100 μg/mL is recommended. Toxic effects that have been described with its use include hepatitis, hemolysis, thrombotic microangiopathy, bone marrow suppression, and fungal pneumonia. Cidofovir is an option for refractory cases. It is a nucleoside analog approved for use in cytomegalovirus retinitis. Its mechanism of action against BKV is poorly understood. It is usually reserved as a last resort for refractory disease. It is primarily excreted by the kidneys but accumulates in tubular epithelial cells with potential for substantial nephrotoxicity, limiting its use in kidney transplant patients. Concomitant administration of probenecid to reduce renal clearance can reduce toxicity. It is usually administered intravenously in doses ranging from 0.25 to 1.0 mg/kg at 1 to 3 weekly intervals [19-22].
Clinicians generally wait for complete viral clearance as demonstrated by BKV non-detection on qPCR to resume IS following BKVN treatment, resulting in the higher incidence of rejection episodes following treatment. Based on evidence from our study data, we believe this is an erroneous treatment endpoint due to a delay between immune reconstitution and viral clearance, which accounts for high frequency of rejection episodes following viral clearance. We found that a progressive increase in cylex level to >200 along with a concomitant decrease in BKV qPCR to <3000 copies/mL was a more accurate marker for treatment endpoint. However, due to the small number of patients studied, it is difficult to draw meaningful conclusions; at best this pilot study is a proof of concept for the need for immune surveillance for BK viremia. It also underscores the need for development of more specific tests, which can monitor BKV specific cell mediated immune response in patients with BK viremia, instead of a non-specific marker of cell mediated immune response.
None.
- Gardner SD, Field AM, Coleman DV, Hulme B (1971) New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet 1: 1253-1257. [Crossref]
- Goudsmit J, Wertheim-van Dillen P, van Strien A, van der Noordaa J (1982) The role of BK virus in acute respiratory tract disease and the presence of BKV DNA in tonsils. J Med Virol 10: 91-99. [Crossref]
- Hirsch HH (2005) BK virus: opportunity makes a pathogen. Clin Infect Dis 41: 354-360. [Crossref]
- Hirsch HH, Knowles W, Dickenmann M, Passweg J, Klimkait T, Mihatsch MJ, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med 347: 488-496. [Crossref]
- Kuypers DR (2012) Management of polyomavirus-associated nephropathy in renal transplant recipients. Nat Rev Nephrol 8: 390-402. [Crossref]
- Hariharan S, Cohen EP, Vasudev B, Orentas R, Viscidi RP, et al. (2005) BK virus-specific antibodies and BKV DNA in renal transplant recipients with BKV nephritis. Am J Transplant 5: 2719-2724. [Crossref]
- Chen Y, Trofe J, Gordon J, Du Pasquier RA, et al. (2006) Interplay of cellular and humoral immune responses against BK virus in kidney transplant recipients with polyomavirus nephropathy. J Virol 80: 3495-3505. [Crossref]
- Drummond JE, Shah KV, Donnenberg AD (1985) Cell-mediated immune responses to BK virus in normal individuals. J Med Virol 17: 237-247. [Crossref]
- Koralnik IJ, Du Pasquier RA, Letvin NL (2001) JC virus-specific cytotoxic T lymphocytes in individuals with progressive multifocal leukoencephalopathy. J Virol 75: 3483-3487. [Crossref]
- Batal I, Zeevi A, Heider A, Girnita A, Basu A, et al. (2008) Measurements of global cell-mediated immunity in renal transplant recipients with BK virus reactivation. Am J Clin Pathol 129: 587-591. [Crossref]
- Binggeli S, Egli A, Dickenmann M, Binet I, Steiger J, et al. (2006) BKV replication and cellular immune responses in renal transplant recipients. Am J Transplant 6: 2218-2219.
- Prosser SE, Orentas RJ, Jurgens L, Cohen EP, Hariharan S (2008) Recovery of BK virus large T-antigen-specific cellular immune response correlates with resolution of bk virus nephritis. Transplantation 85: 185-192. [Crossref]
- Comoli P, Azzi A, Maccario R, Basso S, Botti G, et al. (2004) Polyomavirus BK-specific immunity after kidney transplantation. Transplantation 78: 1229-1232. [Crossref]
- Van Aalderen MC, Heutinck KM, Huisman C, ten Berge IJ (2012) BK virus infection in transplant recipients: clinical manifestations, treatment options and the immune response. Neth J Med 70: 172-183. [Crossref]
- Sharma R, Tzetzo S, Patel S, Zachariah M, Sharma S, et al. (2016) BK Virus in Kidney Transplant: Current Concepts, Recent Advances, and Future Directions. Exp Clin Transplant 14: 377-384. [Crossref]
- Schachtner T, Muller K, Stein M, Diezemann C, Sefrin A, et al. (2011) BK virus-specific immunity kinetics: a predictor of recovery from polyomavirus BK-associated nephropathy. Am J Transplant 11: 2443-2452. [Crossref]
- Josephson MA, Gillen D, Javaid B (2006) Treatment of renal allograft polyoma BK virus infection with leflunomide. Transplantation 81: 704-710. [Crossref]
- Williams JW, Javaid B, Kadambi PV (2005) Leflunomide for polyomavirus type BK nephropathy. N Engl J Med 352: 1157-1158. [Crossref]
- Williams JW, Mital D, Chong A (2002) Experiences with leflunomide in solid organ transplantation. Transplantation 73: 358-366. [Crossref]
- Kadambi PV, Josephson MA, Williams J (2003) Treatment of refractory BK virus-associated nephropathy with cidofovir. Am J Transplant 3: 186-191. [Crossref]
- Keller LS, Peh CA, Nolan J, Bannister KM, Clarkson AR, et al. (2003) BK transplant nephropathy successfully treated with cidofovir. Nephrol Dial Transplant 18: 1013-1014.
- Kuypers DR, Vandooren AK, Lerut E (2005) Adjuvant low-dose cidofovir therapy for BK polyomavirus interstitial nephritis in renal transplant recipients. Am J Transplant 5: 1997-2004. [Crossref]


