The principle of immuno-oncology is to stimulate the patient's natural immune defenses in order to increase the anti-tumor response. With this new therapeutic approach, we are witnessing a paradigm shift: the target is no longer cancer but the immune system, whose potential is released by immunotherapy [1].
The actors of immunity
Cells have multiple mechanisms to prevent the onset of cancer. At the first signs of cell growth deregulation, the NER DNA repair pathway is involved, causing cell senescence and even apoptosis [2]. If this safety system fails, other extrinsic mechanisms intervene, including environmental signals inhibiting tumor growth, but also and especially the immune system by its recognition and ability to eliminate tumor cells to maintain homeostasis. This is the principle of immuno-surveillance [3].
The immune system involves innate immunity cells, such as Natural Killer (NK) cells, dendritic cells and macrophages. These are the first lines of defense of the organization. As for the actors of adaptive immunity, they are involved in tumor control and are able to stimulate or inhibit the development of cancer. These include antigen presenting cells (APCs) that activate T cells (LT) [2,4].
The main effector cells for anti-tumor action are CD8 + T cells, also called cytotoxic lymphocytes. The recognition mechanism of target cells is mediated by a specific receptor on the surface of LTs, the cell receptor (TCR), which recognizes antigenic peptides presented by APCs through major histocompatibility complex molecules (MHCs). CD8 + LTs interact with class I MHCs. They have the ability to recognize "self" and "non-self" antigens. In case of recognition of an antigenic peptide, LT is activated and proceeds to tumor lysis [2].
Tumor antigens
Each tumor carries thousands of different somatic mutations encoding mutated proteins called neo-antigens that are not expressed by healthy cells. Some mutations are considered immunogenic and others are not, depending on their ability to generate neo-epitopes recognizable by LT TCRs.
Melanoma and some tobacco-induced cancers, such as Non-small cell lung cancer (NSCLC), have the distinction of having a strong immunogenic character (Figure 1) and therefore potentially accessible to a treatment that will stimulate immunity [5,6].
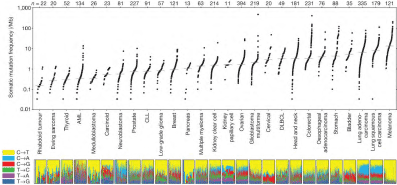
Figure 1.Mutational heterogeneity of cancers, according to Lawrence et al [18] to tumor lysis [15].
The cycle of anti-tumor immunity
The immune cycle in cancer occurs in seven key stages [7], shown in Figure 2, which are potential new therapeutic targets.
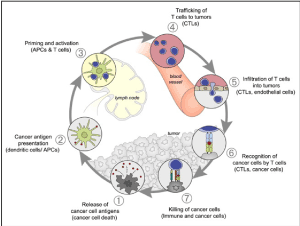
Figure 2.The cycle of anti-tumor immunity, according to Chen et al [7].
The first is the release of antigenic peptides by the tumor cells. These antigens are then captured and treated by the dendritic cells. This recognition of tumor antigens, identified as a threat, may be spontaneous for certain tumors carrying a high mutational load and therefore of tumor neoantigens, whereas it may not appear until after the completion of chemotherapy and / or radiotherapy in other cases. The dendritic cells then present the tumor antigens at LT, through MHC class I (interaction with CD8 +) and class II (interaction with CD4 +). Then comes the initiation and activation phase of the immune cells, which concerns effector T cells (CD4 + and CD8 +), but also regulatory TLs whose role is fundamental. Activated LTs will migrate to the tumor and enter the tumor microenvironment. They recognize the tumor cells and attach to it via the TCR. This step is one of the main steps bypassed by tumor cells to escape the immune system via the PD-1/PD-L1 binding. Sufficient lymphocyte proliferation results in tumor cytolysis.
Mechanisms of carcinogenesis
The oncogenesis processes have several essential mechanisms [8]: unlimited replicative potential, pro-tumor inflammation, invasion capacity and metastatic power, angiogenesis induction, genomic instability, resistance to apoptosis, abnormalities of energy metabolism, uncontrolled proliferation, resistance to proliferation inhibitors. The escape of tumor cells to the immune system is a more recent hypothesis (Figure 3).
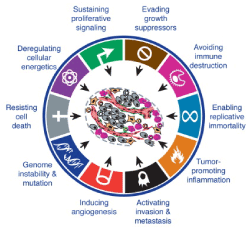
Figure 3. Mechanisms of carcinogenesis, according to Hanahan et al [8].
Principle of immunoediting
Recent data suggest a much more complex role for the immune system - the immunoediting hypothesis [2-4] - where it can both protect and promote tumor growth. Recognition and targeting of tumor cells occurs in three phases that can proceed independently or sequentially (Figure 4) [9].
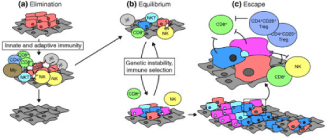
Figure 4. Principle of immunoediting, after Strausberg et al [9] .
Phase 1, Elimination, also called Immunosurveillance. The immune system recognizes the tumor antigens expressed by the cells and eliminates them. At this stage, some cells can acquire mutations allowing them to resist lysis by the immune system.
Phase 2, Balance: some abnormal cells persist but their proliferation and dispersion are controlled by the adaptive immune system. The pressure of the adaptive immunity during this relatively long stage is supposed to allow to "carve" the tumor and thus to select the least immunogenic tumors (loss or masking of tumor antigens) and having accumulated the most favorable mutations for the escape to the immune system. The increase in PD-L1 expression by tumor cells is one example. PD-L1 binds to PD-1 on LTs and thus inhibits their activation.
Phase 3, Exhaust, where when the most aggressive and the least immunogenic residual tumor cells, having acquired enough mutations, manages to multiply and spread. Successive mutations in the surviving abnormal cells give them the ability to metastasize and proliferate indefinitely. Over time, the inhibitory immune responses take over and the immune system goes from anti- to protumoral.
Loss of antigen expression
Some tumor cells are able to repress or completely eliminate MHC molecules, reducing their presentation of tumor-specific antigens [10]. Tumors can also lose their antigens as a result of mutations, and thus remain undetectable by the immune system.
The tumor microenvironment
The tumor microenvironment is particularly complex and some factors influence anti-cancer immunity such as recruitment of immunosuppressive cells and immunosuppressive cytokines [2,4]. Tumors can recruit and direct regulatory LTs to protect them from immune attack because they can prevent the onset of an anti-tumor response by inhibiting LT. Another mechanism is the phenomenon of immune exclusion [11] in which many LT are present at the periphery of the tumor but fail to penetrate, resulting from an abundant stroma (Figure 5) [12].
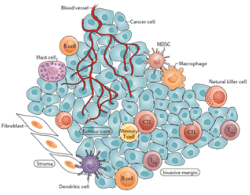
Figure 5.Tumor microenvironment, according to Hack et al [12] .
Immune control points
The immune response is complex and involves a diverse repertoire of immunoregulatory interactions (26). The main ones are shown in Figure 6. It is a series of receptors and ligands carried by the immune cells whose interactions lead either to the stimulation or to the repression of the T lymphocyte activity in order to limit the collateral tissue lesions. during the immune response, that is to say the phenomena of autoimmunity, and thus to maintain immunological homeostasis. These communication systems that modulate lymphocyte activation are called immune control points or immunological checkpoints. They are also expressed by tumor cells, which, in order to escape immune surveillance, can divert them to their benefit, such as PD-1 (Program Death Receptor 1) and CTLA-4 (Cytotoxic T-Lymphocyte-Associated Antigen.4) [14].
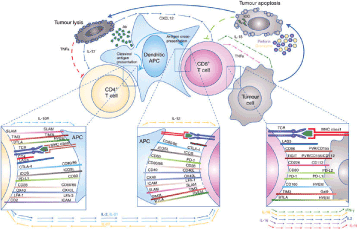
Figure 6.Examples of immune checkpoints, according to Cogdill et al [13].
CTLA-4 and PD-1 are the first two immunological control points to have been thoroughly evaluated in the context of cancer immunotherapy. These are two immune axes among many others that differ in the manner and level at which they negatively regulate the immune system [15].
CTLA-4 is an inhibitory molecule expressed by LTs during their activation in secondary lymphoid organs. It negatively regulates their activation and differentiation into LT effectors by competing with the CD28 costimulatory molecule to bind B7.1 (or CD80) and B7.2 (or CD86) ligands. CTLA-4 has a greater affinity for these ligands and counteracts the activating effect of CD28, resulting in lymphocyte inhibition [16,17].
PD-1 is strongly expressed on tumor infiltrating lymphocytes (TIL) in the effector phase and serves to inhibit LT activity when ligated by its ligands [15]. In peripheral tissues, tumor cells and other tumor microenvironment cells overexpress the PD-1, PD-L1 and PD-L2 ligands, which are thought to protect tumor cells from immune destruction. This binding will inhibit LT, allowing immuno-subversion of cancer cells that escape the immune response [18].
PD-L1 is an emerging predictive biomarker in oncology [19]. It is expressed on the surface of tumor cells in many types of tumors and may indicate tumor inhibition of the immune response. His research is done by immunohistochemistry; we speak of positive labeling when 1 to 100% of the tumor cells of a sample are concerned [19]. The search for the level of expression of PD-L1 in advanced NSCLC is currently systematic, from the first tumor biopsy, to guide the therapeutic decision.
Understanding the mechanism of action of inhibitory checkpoints, anti-CTLA4, anti-PD-L1 and anti-PD-L1 paves the way for the development of new active molecules on a larger number of epithelial tumors.
- Brahmer JR (2013) Harnessing the immune system for the treatment of non-small-cell lung cancer. J Clin Oncol 31: 1021-1028. [Crossref]
- Owen JA, Punt J, Stranford SA, Jones PP, Kuby J (2013) Kuby immunology. (7thedtn). WH Freeman, New York. Pp: 692.
- Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331: 1565-1570. [Crossref]
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ (2011) Natural innate and adaptive immunity to cancer. Annu Rev Immunol 29: 235-271. [Crossref]
- Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, et al. (2013) Mutational heterogeneity in cancer and the search for new cancer genes. Nature 499: 214-218. [Crossref]
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, et al. (2015) Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 348: 124-128. [Crossref]
- Chen DS, Mellman I (2013) Oncology meets immunology: the cancer-immunity cycle. Immunity 39: 1-10. [Crossref]
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646-674. [Crossref]
- Strausberg RL (2005) Tumor microenvironments, the immune system and cancer survival. Genome Biol 6: 211. [Crossref]
- Ahmad M, Rees RC, Ali SA (2004) Escape from immunotherapy: possible mechanisms that influence tumor regression/progression. Cancer Immunol Immunother 53: 844-854. [Crossref]
- Lopez A (2018) Can we optimize immune checkpoint inhibitors efficacy in digestive oncology? Gastro Med Res 1: GMR.000508.
- Hackl H, Charoentong P, Finotello F, Trajanoski Z (2016) Computational genomics tools for dissecting tumour-immune cell interactions. Nat Rev Genet 17: 441-458. [Crossref]
- Cogdill AP, Andrews MC, Wargo JA (2017) Hallmarks of response to immune checkpoint blockade. Br J Cancer 117: 1-7. [Crossref]
- Momtaz P, Postow MA (2014) Immunologic checkpoints in cancer therapy: focus on the programmed death-1 (PD-1) receptor pathway. Pharmgenomics Pers Med 7: 357-365. [Crossref]
- Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, et al. (2005) CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 25: 9543-9553. [Crossref]
- Schwartz RH (1992) Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell 71: 1065-1068. [Crossref]
- Egen JG, Allison JP (2002) Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity 16: 23-35. [Crossref]
- Qin A, Coffey DG, Warren EH, Ramnath N (2016) Mechanisms of immune evasion and current status of checkpoint inhibitors in non-small cell lung cancer. Cancer Med 5: 2567-2578. [Crossref]
- Patel SP, Kurzrock R (2015) PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther 14: 847-856. [Crossref]






