Abstract
Aim: Study was to characterize by immuno-fluorescence, the expression of the serotonin reuptake transporter protein (SERT); 5-HT1B, 5-HT2A, and 5-HT2B serotonin receptors; as well as type 1, and type 2 tryptophan 5-hydroxylase enzymes (TPH1 and TPH2), on the left ventricular free wall and on the interventricular septum of normal hearts of patients who died from non-cardiovascular diseases.
Methods: Six different samples taken from the left ventricular free wall and the interventricular septum of six different normal hearts were analyzed by immune-histochemistry by means of specific antibodies.
Results: There were found positive immune-reactive cardiomyocytes for SERT and 5-HT1B, 5-HT2A, and 5-HT2B predominantly in the interventricular septum in contrast to the left ventricular free wall. Likewise, immunoreactivity for TPH1 and TPH2 was mainly positive in the interventricular septum in contrast to the left ventricular free wall.
Conclusions: On these bases, the presence of SERT, 5-HT1B, 5-HT2A, and 5-HT2B, and both TPH1, and TPH2, suggests the possibility that cardiomyocytes could use, synthesize, release, and reuptake 5-HT, which suggests an intrinsic serotonergic system involved in the autocrine and paracrine modulation of human heart function.
Key-words
Cardiomyocytes, serotonin, SERT, tryptophan-5-hydroxylase, serotonergic receptors
Introduction
Serotonin (5-HT) exerts its influence in neuronal and non-neuronal systems, leading to a variety of functions like neurotransmission, signal neuromodulation or even triggering mitogen activated protein-kinase activity [1-5]. Synthesis of this molecule begins with the hydroxylation of L-tryptophan (L-Trp) by means of a tryptophan-5-hydroxylase (TPH), which is a limiting enzyme [6,7]; thus, 5-HT production depends on the availability of L-Trp and the activity of TPH [8,9]. There are two different TPH isoforms: TPH1 whose expression predominates in peripheral tissues like pineal gland, gut, heart, thymus, spleen, lungs and TPH2 which is found in serotonergic neurons of the brain stem, taking into account that recently, it has also been found in the gut, pancreas and heart [10-14]. TPH1 in fact, seems to lead to the synthesis of 5-HT in adult as well as in the developing brain [15]. Meanwhile plasma 5-HT is synthesized in chromaffin cells of the gut; taking into account that platelets are their main place of storage [11,16-18]. Nevertheless, 5-HT is also synthesized in the heart, pancreas, and adipose tissue, where it plays an important role in cell immune response [12,13,19].
A group of membrane receptors are able to transduce 5-HT signal, whenever this modulator interacts with the receptor area. Up to date, serotonin receptors 5-HT1A, 5-HT2A, 5-HT3, 5-HT4, and 5-HT7, besides being shown on the surface of cardiomyocytes and on the ending myocardial nerves, they are also involved in the regulation of cardiovascular function [20-25]. Likewise, SERT has also been shown in hearts during the fetal stage, probably because of the role 5-HT plays in heart development; and also in adult cardiomyocytes structure, including interstitial cells of heart valves, probably triggering rebuilding heart structure [26-29]. 5-HT seems to play an important role in the physiopathology of valve dysfunction, since it can be triggered by the use of 5-HT2B antagonist or SERT inhibitors or even, during the development of a carcinoid syndrome [26,30-34]. Likewise, the activation of heart 5-HT2B besides triggering collagen synthesis in fibroblasts, it also may produce fibroblastic mitogenesis and subendocardial fibrosis [26,30]. It has also been demonstrated that 5-HT is able to trigger a positive inotropic effect on cardiomyocytes of animal models with cardiac insufficiency, through the activation of 5-HT2A, and 5-HT4, which lead to the phosphorylation of the light chain of myosin [25,35]. Furthermore, during cardiogenesis a number of mast cells capable of releasing 5-HT appear in the heart tissue, and TPH starts to express itself not only during this stage, but in the adult heart, especially in the interstitial cells of the heart valves [36,37]. On these bases, we tried to demonstrate the expression of SERT, 5-HT1B, 5-HT2A, and 5-HT2B; as well as TPH1 and TPH2 in normal human hearts, with the idea that 5-HT plays an important role in the autocrine and paracrine modulation of the cardiovascular system.
Material and Methods
Six different samples of human hearts donated by the pathology department of the Mexican General Hospital of the Secretary of Health were used in this study. All of the samples of hearts were obtained from patients who died from non-cardiovascular diseases. Heart tissue was subjected to an immunohistochemically technique to identify the expression of SERT, 5-HT1B, 5-HT2A, and 5-HT2B; as well as TPH1 and TPH2. Tissue was cut in slices of 5 µM and put in electro-charged slides. Then, it was carried an antigenic unmask by means of a 0.1 M citrate buffer solution, pH 6.0 (DECLERE, Biosciences), followed by the inhibition of endoperoxidase activity with H2O2 to 3%; after washing the tissue, it was blocked with gout serum to 10% and Triton X 100 to 0.01%, and diluted in PBS for 30 minutes. After this, slides were incubated with specific primary monoclonal antibodies against TPH1 (Gene Tex International Corp), and TPH2 (Merck-Millipore USA); and polyclonal antibodies against SERT (Chemicon, USA) and against 5-HT1B, 5-HT2A, and 5-HT2B (Santa Cruz, Biotechnology Inc, USA) at 1:500 in a 0.1 M PBS solution, pH 7.4, and Triton X 100 to 0.3%. After 24 hours, samples were washed with PBS-Tween at 0.1%, and then subjected to incubation with Alexa Fluor 546 anti-rabbit diluted to 1:100 [38]. Eventually, cuts were put on Vectashield for immuno-fluorescence, and analyzed under an Olympus BX51 fluorescence microscope, adapted with an Infinity 1 Lumera digital camera with a 40X objective.
Results
Figure 1 shows heart tissue cuts from the left ventricular free wall (A) and from the interventricular septum (B). Positive immune-reactive cardiomyocytes for TPH1 appeared in both regions; taking into account that the majority of the cardiomyocytes were not reactive, and that the immune-reactivity was located in their intracytoplasmic granules. Nevertheless, the expression of TPH1 was more important in the interventricular septum in contrast to the left ventricular free wall (Figure 1B).
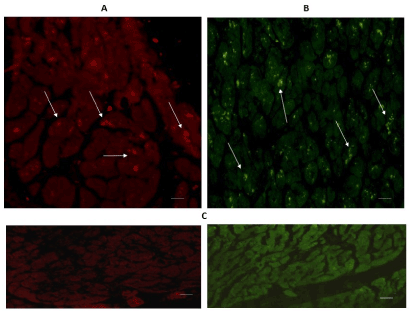
Figure 1. Cross-sectional human heart microphotographs. A. Left ventricular free wall; B. Interventricular septum with positive immune-reactive cardiomyocytes for TPH1. Myocardium tissue was incubated with specific monoclonal antibodies (dilution 1:500), and immunereactivity was carried out with Alexa Fluor 546 anti-Rabbit (1:100). Arrows show cytoplasmic immunoreactivity. C. Negative control. Scale 40 X —5 µM.
Immune-reactive cardiomyocytes for TPH2 were also positive in both regions as it is shown in Figures 2A & 2B. TPH2 expression was located in the cytoplasm of cardiomyocytes, especially in cardiomyocytes of the interventricular septum (Figure 2B).
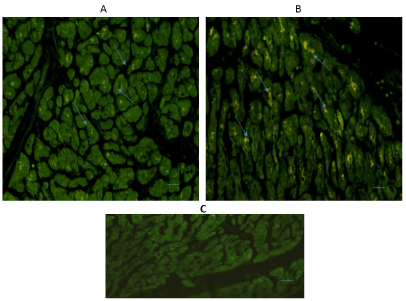
Figure 2. Cross-sectional human heart microphotographs. A. Left ventricular free wall; B. Interventricular septum showing immuno-positive cardiomyocytes for TPH2. Tissue cuts were incubated with specific monoclonal antibodies for the isoform (dilution 1:500), and Immunoreactivity was detected with Alexa Fluor 546 anti-Rabbit (1:100). Arrows show cytoplasmic immunoreactivity. C) Negative control. Scale 40 X —5 µM.
SERT expression also appeared in both regions, considering that immune-reactivity was located in the intracytoplasmic granules of cardiomyocytes, as it can be seen in Figures 3A & 3B.
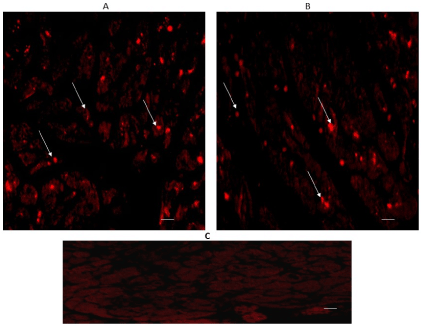
Figure 3. Cross-sectional human heart microphotographs. A. Left ventricular free wall. B. Interventricular septum showing immune-positive cardiomyocytes for SERT. Tissue cuts were incubated with protein-specific polyclonal antibodies (dilution 1:500), and immunoreactivity was detected with Alexa Fluor 546 anti-Rabbit (1:100). Arrows show cytoplasmic immunoreactivity conglomerates. C) Negative control. Scale 40 X, —5 µM.
Figure 4 shows the expression of 5-HT1B in the left ventricular free wall (A) and in the interventricular septum (B). Immunoreactivity was diffusely located in the cytoplasm of cardiomyocytes of both regions (Figure 4 Arrows).
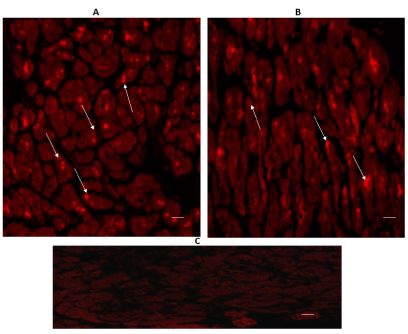
Figure 4. Cross-sectional human heart microphotographs. A. Left ventricular free wall. B. Interventricular septum showing immuno-positive cardiomyocytes for 5-HT1B. Tissue cuts were incubated with polyclonal specific-protein antibodies (dilution 1:500), and immunoreactivity was detected with Alexa Fluor 546 anti-Rabbit (1:100). Arrows show cytoplasmic immunoreactivity conglomerates.C. Negative control. Scale 40 X, —5 µM.
The expression of 5-HT2A and 5-HT2B can be seen in Figures 5 & 6. Immuno-reactivity was positive in some cardiomyocytes of both regions: the left ventricular free wall (A), and the intraventricular septum (B). The expression of 5-HT2B was more important in the interventricular septum.
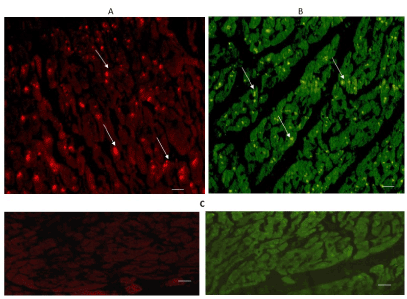
Figure 5. Cross-sectional human heart microphotographs. A. Left ventricular free wall. B. Interventricular septum showing immuno-positive cardiomyocytes for 5-HT2A. Tissue cuts were incubated with polyclonal specific-protein antibodies (dilution 1:500), and immunoreactivity was detected with Alexa Fluor 546 anti-Rabbit (1:100). Arrows show cytoplasmic immunoreactivity conglomerates.C. Negative control. Scale 40 X, —5 µM.
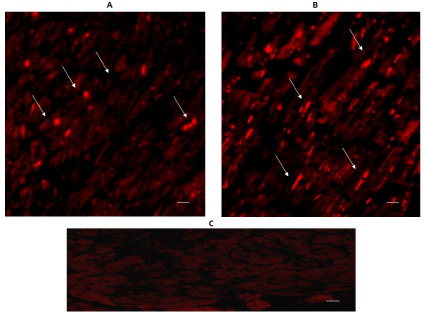
Figure 6. Cross-sectional human heart microphotographs. A. Left ventricular free wall. B. Interventricular septum showing immuno-positive cardiomyocytes for 5-HT2B. Tissue cuts were incubated with polyclonal specific-protein antibodies (dilution 1:500), and immunoreactivity was detected with Alexa Fluor 546 anti-Rabbit (1:100). Arrows show cytoplasmic immunoreactivity conglomerates.C. Negative control. Scale 40 X, —5 µM.
Discussion
The main objective of this research was to demonstrate the presence of SERT, 5-HT1B, 5-HT2A, and 5-HT2B; as well as TPH1 and TPH2 in the left ventricular free wall and in the interventricular septum of normal hearts of patients who died from non-cardiovascular diseases. Thus, with our results we support the idea that human cardiomyocytes own molecular and biochemistry mechanisms to synthesize, use, release, and re-uptake 5-HT, since they express not only 5-HT receptors, but SERT and both isoforms of TPH. Nevertheless 5-HT receptors, SERT and the isoforms of TPH were found in the left ventricular free wall as well as in the interventricular septum, they clearly predominated in the former. This, could suggest for one thing, the possibility of the existence of different cardiomyocytes subtypes within this region, which some of them would be capable of expressing TPH, while others could not do it, and for another, that this region produces therefore, more 5-HT than the left ventricular free wall, which could be used to modulate ventricular activity, since it can lead to septum stiffness.
It was also shown the expression of SERT in cardiomyocytes of the left ventricular free wall and the interventricular septum of human hearts, as it can be seen in Figure 3. In fact, SERT, a protein involved in tissue and plasma 5-HT removing, has been found during the fetal stage in cardiomyocytes as well as in the endocardium and in the endothelium of lung capillaries and coronary arteries; which suggests the possibility that these cells own a great capacity to re-uptake blood stream 5-HT to be catabolized [17,18,26,28,29,31,39]. Thus, it seems that besides the function of the endothelium of lung and coronary arteries; cardiomyocytes activity also shows a 5-HT re-uptake function by means of SERT, regulating 5-HT influence on heart activity.
Of special interest was the expression of 5-HT1B, 5-HT2A, and 5-HT2B in cardiomyocytes of the left ventricular free wall and in the interventricular septum, since it has been demonstrated in experimental models the involvement of 5-HT2A in the induction of myocardial contractility during acute heart insufficiency [25], the development of structural and functional myocardial abnormalities in absence of 5-HT2B during cardiogenesis [40-43], myocardial hypertrophy with the over-expression of 5-HT2B [44], or the triggering of fibroblast proliferation by 5-HT1B in the surroundings of heart valves [45].
Overall, this study demonstrates the expression of SERT, 5-HT1B, 5-HT2A, and 5-HT2B; as well as TPH1 and TPH2 in the left ventricular free wall and mainly in the interventricular septum of normal human hearts. Taking into account these findings, we propose the existence of an intrinsic serotonergic system in human hearts, never described up to now. As a matter of fact, there is enough evidence to support human cardiomyocytes as the biochemical system capable of synthesize, release, use and re-uptake 5-HT, giving rise to an intrinsic serotonergic system involved in the autocrine and paracrine modulation of human heart function.
Acknowledgments
This study was carried out with funding provided by the Instituto Mexicano del Seguro Social (grant FIS/IMSS/PROT/G15/1404) and CONACYT of MEXICO
Conflict of interest statement
The authors declare that they have no conflicts of interest concerning this article
References
- Lauder JM (1993) Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci 16: 233-239. [Crossref]
- Jacobs BL, Azmitia EC (1992) Structure and function of the brain serotonin system. Physiol Rev 72: 165-220. [Crossref]
- Mercado R, Hernandez J (1992) A molecular recognizing system of serotonin in rat fetal axonal growth cones; uptake and high affinity binding. Dev Brain Res 69: 133-137. [Crossref]
- Gaspar P, Lillesaar C (2012) Probing the diversity of serotonin neurons.Philos Trans R Soc Lond B Biol Sci367: 2382-2394. [Crossref]
- Kiyasova V, Gaspar P (2011) Development of raphe serotonin neurons from specification to guidance.Eur J Neurosci34: 1553-1562. [Crossref]
- Boadle-Biber MC (1993) Regulation of serotonin synthesis.Prog Biophys Mol Biol60: 1-15. [Crossref]
- Walther DJ, Bader M (2003) A unique central tryptophan hydroxylase isoform. Biochem Pharmacol 66: 1673-1680. [Crossref]
- Tagliamonte A, Biggio G, Vargiu L, Gessa GL (1973) Free tryptophan in serum controls brain tryptophan level and serotonin synthesis.Life Sci II12: 277-287. [Crossref]
- McmenamY RH, Oncley JL (1958) The specific binding of L-tryptophan to serum albumin.J Biol Chem233: 1436-1447. [Crossref]
- Patel PD, Pontrello C, Burke S (2004) Robust and tissue-specific expression of TPH2 versus TPH1 in rat raphe and pineal gland.Biol Psychiatry55: 428-433. [Crossref]
- O'Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF (2015) Serotonin, tryptophan metabolism and the brain-gut-microbiome axis.Behav Brain Res277: 32-48. [Crossref]
- Paulmann N, Grohmann M, Voigt JP, Bert B, Vowinckel J, et al. (2009) Intracellular Serotonin Modulates Insulin Secretion from Pancreatic beta-Cells by Protein Serotonylation. PLoS Biol 7: e1000229. [Crossref]
- Manjarrez GG, Neri TG, Boyzo-MA, Hernández-Rodríguez J (2015) Characterization of an intrinsic serotonergic system in rat heart. Glo Adv Res J Med Med Sci 4: 083-091.
- Amireault P, Sibon D, Côte F (2013) Life without Peripheral Serotonin: Insights from Tryptophan Hydroxylase 1 Knockout Mice Reveal the Existence of Paracrine/Autocrine Serotonergic Networks. ACS Chem Neurosci 4: 64-71. [Crossref]
- Nakamura K, Sugawara Y, Sawabe K, Ohashi A, Tsurui H, et al. (2006) Late developmental stage-specific role of tryptophan hydroxylase 1 in brain serotonin levels. J Neurosci 26: 530-534. [Crossref]
- Sumara G, Sumara O, Kim JK, Karsenty G (2012) Gut-derived serotonin is a multifunctional determinant to fasting adaptation.Cell Metab16: 588-600. [Crossref]
- Mercado CP, Kilic F (2010) Molecular mechanisms of SERT in platelets: regulation of plasma serotonin levels. Mol Interv 10: 231-241. [Crossref]
- Manjarrez GG, Herrera MR, Neri TG, Hernández-Rodríguez J (2014) Serotonin levels in plasma and platelets of adolescents with type 1 diabetes. Glo Adv Res J Med Med Sci 3: 267-274.
- Haub S, Ritze Y, Ladel I, Saum K, Hubert A, et al. (2011) Serotonin receptor type 3 antagonists improve obesity-associated fatty liver disease in mice. J Pharmacol Exp Ther 339: 790-798. [Crossref]
- Hoyer D, Hannon JP, Martin GR (2002) Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 71: 533-554. [Crossref]
- Nebigil CG, Maroteaux L (2001) A novel role for serotonin in heart.Trends Cardiovasc Med11: 329-335. [Crossref]
- Frishman WH, Grewall P (2000) Serotonin and the heart.Ann Med32: 195-209. [Crossref]
- Ramage AG, Villalón CM (2008) 5-hydroxytryptamine and cardiovascular regulation.Trends Pharmacol Sci29: 472-481. [Crossref]
- Côté F, Thévenot E, Fligny C, Fromes Y, Darmon M, et al. (2003) Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function.Proc Natl Acad Sci U S A100: 13525-13530. [Crossref]
- Qvisgstad E, Sajaastad I, Brattelid T, Nunn C, Swift F, et al. (2005) Dual serotonergic regulation of ventricular contractile force through 5-HT2A and 5-HT4 receptors induced in the acute failing heart. Circ Res 97: 268-276. [Crossref]
- Mekontso-Dessap A, Brouri F, Pascal O, Lechat P, Hanoun N, et al. (2006) Deficiency of the 5-hydroxytryptamine transporter gene leads to cardiac fibrosis and valvulopathy in mice. Circulation 113: 81-9. [Crossref]
- Gustafsson BI, Tømmeras K, Nordrum I, Loennechen JP, Brunsvik A, et al. (2005) Long-term serotonin administration induces heart valve disease in rats. Circulation 111: 1517–1522. [Crossref]
- Sari Y, Zhou FC (2003) Serotonin and its transporter on proliferation of fetal heart cells.Int J Dev Neurosci21: 417-424. [Crossref]
- Pavone LM, Mithbaokar P, Mastellone V, Avallone L, Gaspar P, et al. (2007) Fat map of serotonin Transporter-Expressing Cells in Developing Mouse Heart. Genesis 45: 689-695. [Crossref]
- Elangbam CS, Lightfoot RM, Yoon LW, Creech DR, Geske RS, et al. (2005) 5-Hydroxytryptamine (5HT) receptors in the heart valves of cynomolgus monkeys and Sprague-Dawley rats.J Histochem Cytochem53: 671-677. [Crossref]
- Levy RJ (2006) Serotonin transporter mechanisms and cardiac disease. Circulation 113: 2-4. [Crossref]
- Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, et al. (1997) Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med 337: 581–588. [Crossref]
- Fitzgerald LW, Burn TC, Brown BS, Patterson JP, Corjay MH, et al. (2000) Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine.Mol Pharmacol57: 75-81. [Crossref]
- Robiolio PA, Rigolin VH, Wilson JS, Harrison JK, Sanders LL, et al. (1995) Carcinoid heart disease: correlation of high serotonin levels with valvular abnormalities detected by cardiac catheterization and echocardiography. Circulation 92: 790–795. [Crossref]
- Brattelid T, Qvigstad E, Moltzau LR, Bekkevold SV, Sandnes DL, et al. (2012) The cardiac ventricular 5-HT4 receptor is functional in late foetal development and is reactivated in heart failure.PLoS One7: e45489. [Crossref]
- Manjarrez-Gutiérrez G, Camacho-Calderón N, Mercado-Camargo R, Boyzo-Montes de Oca A, Arvizu-Flores A, et al. (2009) Characterization of serotonergic cells in fetal heart tissue.Cir Cir77: 395-400. [Crossref]
- Disatian S, Lacerda C, Orton EC (2010) Tryptophan hydroxylase 1 expression in increased in phenotype-altered canine and human degenerative myxomatous mitral valves. J Heart Valve Dis 19: 71-78. [Crossref]
- Naish JS, Boenisch AJ, Farmilio U (1989) Handbook of Immunochemical Staining Methods, Dako, Carpinteria, CA.
- Murphy DL, Lesch KP (2008) Targeting the murine serotonin transporter: insights into human neurobiology. Nat Rev Neurosci 9(2):85-96. [Crossref]
- Nebigil CG, Choi DS, Dierich A, Hickel P, Le Meur M, et al. (2000) Serotonin 2B receptor is required for heart development.Proc Natl Acad Sci U S A97: 9508-9513. [Crossref]
- Nebigil CG, Etienne N, Schaerlinger B, Hickel P, Launay JM, et al. (2001) Developmentally regulated serotonin 5-HT2B receptors.Int J Dev Neurosci19: 365-372. [Crossref]
- Choi DS, Ward SJ, Messaddeq N, Launay JM, Maroteaux L (1997) 5-HT2B receptor-mediated serotonin morphogenetic functions in mouse cranial neural crest and myocardiac cells. Development 124: 1745-1755. [Crossref]
- Nebigil CG, Hickel P, Messaddeq N, Vonesch JL, Douchet MP, et al. (2001) Ablation of serotonin 5-HT(2B) receptors in mice leads to abnormal cardiac structure and function.Circulation103: 2973-2979. [Crossref]
- Nebigil CG, Jaffre F, Messaddeq N, Hickel P, Monassier L, et al. (2003) Overexpression of the serotonin 5-HT2B receptor in heart leads to abnormal mitochondrial function and hypertrophy. Circulation 107: 3223-3229. [Crossref]
- Seuwen K, Magnaldo I, Pouysségur J (1988) Serotonin stimulates DNA synthesis in fibroblasts acting through 5-HT1B receptors coupled to a Gi-protein.Nature335: 254-256. [Crossref]






