Leptomeningeal disease (LMD), also known as leptomeningeal carcinomatosis, is a rare cancer complication, in which malignant cells infiltrate the layers of the central nervous system (CNS), known as meninges. Magnetic Resonance Imaging (MRI) demonstrate multiple masses within the subarachnoid space or diffuse leptomeningeal enhancement. We present six clinical cases with the MR image of LMD, five of them with medullastomas (four clinical cases in adulthood with late LMD and one in childhood with an initial LMD). One of the clinical cases is in a woman 10 years after the diagnosis of HER2 positive invasive breast cancer after prolonged complex treatment.
We emphasize the significance of MRI in the diagnostics and staging of meduloblastoma in childhood and adulthood, directly related to the necessary complex treatment, including neurosurgery, radiotherapy and chemotherapy. Brain and spinal cord MRI is required in the diagnosis of late LMD in the case of extracranial solid neoplasms. Radiation therapy (RT) has a different target volume and necessary radiation doses, depending on the histological appearance of tumor cells, as well as on the volume and localization of LMD.
leptomeningeal disease, MRI, meduloblastoma, imaging, radiotherapy, complex treatment
Leptomeningeal disease (LMD), also known as leptomeningeal carcinomatosis, is a rare cancer complication, in which malignant cells infiltrate the layers of the central nervous system (CNS) [1-3]. Common neoplastic etiologies of LMD include breast (41%), lung (24%), gastrointestinal tract (13%), melanoma (12%), primary CNS cancers (such as medulloblastoma, ependymoma, pineoblastoma, primitive neuroectodermal, or primary CNS lymphoma), NH lymphoma, leukemia, and multiple myeloma [4-6]. Today, it is known that LMD occurs in ~5% of all cancer patients [3,7,8]. MRI and CT demonstrate multiple masses within the subarachnoid space, hydrocephalus without a discernible cause, or diffuse leptomeningeal enhancement [9]. In current practice, diagnosis of LMD is also often made without cerebrospinal fluid (CSF) analysis, using gadolinium-enhanced magnetic resonance imaging (gdMRI) of the brain and/or spinal cord, which is reported to have a sensitivity and specificity of approximately 75% [10,11]. The frequency of LMD-related MRI abnormalities for solid tumors on neuroaxis imaging varies from 56% to 65%, depending on the primary [12]. In this article, we present the importance of the brain and spinal cord gdMRI for the determination of the radiotherapy target volume in the complex treatment of initial and late LMD in medulloblastomas and solid cancers.
Clinical case № 1
We present a young woman 37 years old with medulloblastoma, diagnosed 7 years ago. Subtotal tumour extirpation was carried out, followed by craniospinal radiation therapy (CSRT) with TD-36 Gy and boost in cerebellum upto TD-54 Gy with daily dose (DD) 2 Gy in 27 fractions. After four years of disease-free survival, three local relapses had been manifested (2017, 2018 and 2019), which have been surgically removed. Since the operation of the third relapse, 6 courses combined chemotherapy with Etoposide and Carboplatin have been conducted. In the background of the last chemotherapeutic course, MRI of the craniospinal axis visualizes diffuse subependymal periventriculare metastases, combined with leptomeningeal metastases in the cerebellum and the entire spinal axis (Figures 1 and 2).
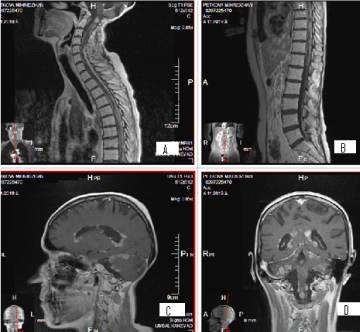
Figure 1. A, B/ SAG T1 FLAIR MR postcontrast images showing hyperintense leptomeningeal spinal lesions; C, D/ SAG T1 FLAIR MRI and C/COR T1 FLAIR MRI-postcontrast images showing hyperintense nodular subependymal and leptomeningeal brain lesions, predominantly infratentorial, with coverage of the cerebellum and medulla oblongata
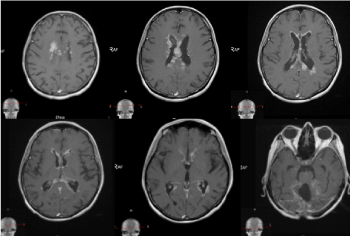
Figure 2. AX T1 FLAIR MR postcontrast images showing hyperintense subependymal periventricular and leptomeningeal brain lesions in triple local relapsed medulloblastoma
The re-CSRT with linear accelerator was conducted with the Volume Modulated Arc Therapy (VMAT) method in the spinal cord upto TD-24 Gy; cauda equina upto TD-28 Gy; in cerebellum upto TD-24 Gy; the two hemispheres upto TD-26 Gy and paraventricular upto TD-30 Gy with DD-1.8 Gy/17 fractions. Three months after the completion of the re-CSRT, we conducted an MRT of the brain and cervical spinal cord, which reported a lack of subependimal periventricular and leptomeningeal metastases in the brain, cerebellum and cervical spinal axis (Figures 3 and 4).
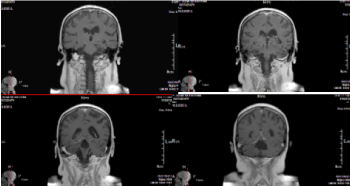
Figure 3. COR T1 FLAIR MR postcontrast images without subependymal and leptomeningeal brain lesions, three months after the re-CSRT
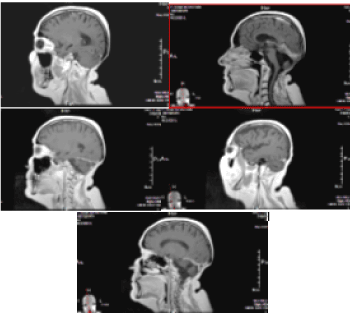
Figure 4. SAG T1 FLAIR MR postcontrast images without subependymal and leptomeningeal brain lesions, three months after the re-CSRT
The patient was directed to conducting chemotherapy and target therapy.
Clinical case № 2
It concerns a female patient of 48 years of age diagnosed with a 10-year-old invasive intraductal carcinoma of the right mammary glands /рТ2N0M0, G2, positive estrogen and progesterone receptors, HER2/+ + +. Surgery (quadrantectomy with axillary dissection), adjuvant chemotherapy, radiotherapy of right mammary gland with operative scarring to OOD 50 Gy, endocrine therapy, targeted therapy with Herepectin was conducted. Two years after the diagnosis, local relapse is manifests, surgically removed by a simple mastectomy with subsequent healing PCT, herepectin and endocrine therapy. In the next 8 years, a consistent solitar liver and pulmonary metastases are diagnosed sequentially, which are surgically removed. Patient aminess continues systemic chemotherapy (SC) and target therapy. On the occasion of an epileptic seizure, after the CT of the brain with venous contrast, the infra- and subtenorial metastasis in the cerebrum is established. The MRI of neuroaxial reports leptomeningeal brain metastases, mainly infratentorial in the cerebellum and medulla oblongata, with mild compression of the brainstem. The spinal axis is not engaged (Figures 5 and 6).
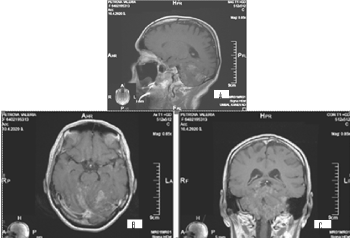
Figure 5. A/SAG T1 FLAIR MRI; B/ AX T1 FLAIR MRI and C/COR T1 FLAIR MRI- postcontrast images showing hyperintense leptomeningeal brain lesions, mainly infratentorial in the cerebellum and medulla oblongata, with mild compression of the brainstem
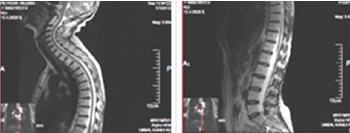
Figure 6. SAG T2 MR images without pathological lesions in the spinal cord
It was assessed for whole brain radiotherapy (WBRT) to ТD 40 Gy with DFD 2 Gy, which is currently being conducted, after which target therapy will be conducted.
Clinical case № 3
A child of 4 years without family history with histological proven desmoplastic histological variant of the medulloblastoma. The disease begins in March 2012 with a progressively increasing headache in the frontal region, repeated daily vomiting, staggering gait and aggressive behavior change. After 3 months, ocular neurological changes to the left eye are manifested. After the CT of the brain a tumor formation in the area of vermis with compression of 4th ventricle and obstructive hydrocephalus is diagnosed. In July 2012, an apparent total tumour extirpation was performed without a ventriculo-peritoneal shunt. After 1 month on the occasion of the cerebrospinal fluid (CSF) from the operative wound, a spinal drainage was placed, accompanied by a dura mater plastic with fascia lata graft. The MRI of the cerebrum and cervical myelon/24.09.2012 -Symmetrically moderately dilated ventricular system. Discrete hyperintense foci in the white brain periventrikular around the anterior and posterior horns, as in transependides of the licvour migration. The 4th ventricle has deformed outlines, with dimensions 16.8 mm. After contrasting perifocally in the dorsal part of the 4th ventricle, a poorly limited hyperintense foci with with dimensions 18/9 mm in a sagittal plan is formed. On T2 FLAIR images – hyperintense zones with nodular and linear form with meningeal localization. The lesions are located on the left parietal and parasagittal, on the right occipital and along the upper cerebellar sulcus. After contrasting, they increase their moderate signal intensity. At the cervical myelon level, after contrasting, there are discrete punctate zones of increased intensity. Occipital CSF fluid collection with dimensions 44/12mm is reported.
Conclusion
MR image for moderate internal hydrocephalus. Residual formation of 4th ventricle, supra and infratentorial leptomeningeal metastases in the brain and at the level cervical spinal cord (Figure 7).
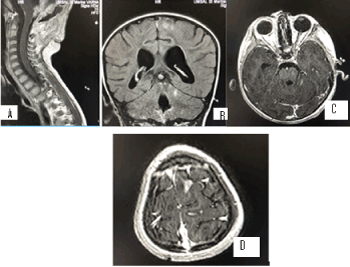
Figure 7. А/ SAG T1+C МR postcontrast image through the brainstem, which shows a residual formation around IV ventricle, in places with leptomeningeal metastases, along the cervical myelon; B/ COR T2 FLAIR MR image through IV ventricle, showing single hyperintense leptomeningeal lesions, supra- and infratentorial; C/ AX 3DT1+C postcontrast image with single contrast-enhancing leptomeningeal lesions on folia cerebelli; D/ AX 3DT1+C postcontrast image with single contrast-enhancing leptomeningeal lesions in the left top fronto-parietal cerebral region
The MRI of the thoracic and lumbar department of the spine does not take into account the areas of the metastases. From the cytology of cerebrospinal fluid, small cohesive cell groups containing small to medium-sized tumor cells with hyperchromatic nuclei, scarce cytoplasm and the presence of nuclear molding are reported.
Conclusion
CSF metastases from Medulloblastoma. After the diagnosis of metastatic disease, a complex postoperative treatment, including 5 courses SC with Etoposide and Carboplatinum, control MRI of neuroaxis, followed by CSRT, is evaluated. Control MR image of the cerebrum, cervical and thoracic myelon/18.12.2012: MP data on mild internal hydrocephalus. Changes with the dural localization dorsal from 4th ventricle, possibly an expression of postoperative changes. Reduction of leptomeningeal metastases (LM) with the presence of a single subtentorial zone for leptomeningeal metastatic involvement of the level medulla oblongata (Figure 8). The control cytological study of cerebrospinal fluid in January 2013 - the presence of tumour cells.
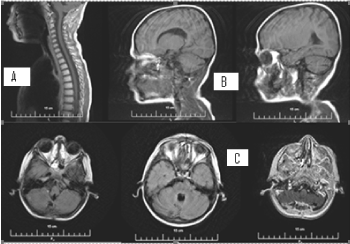
Figure 8. A/SAG T1+C MR postcontrast images of the cervical and thoracic myelon without visible leptomeningeal spinal metastases; B, C/ SAG T1, AX T1, AX 3DT1+C MR postcontrast images of the cerebrum with the presence of a single subtentorial zone for leptomeningeal metastatic involvement of the level medulla oblongata
After 5 cycle SC was conducted CSRT to total dose (TD) 34,5Gy with deily fraction dose (DFD) 1.5 Gy in the craniospinal axis with boost in the posterior cranial fossa (PCF) to TD 44 Gy with DFD 1,8 Gy (planned TD 55 Gy). Radiotherapy was discontinued on the abovementioned dose, due to thrombocytopenia. From CSF study/March 2013, tumour cells are missing. After three further cycle SC with Etoposide and Carboplatin of control MRT/ 05.06.2013 -Symmetrically slightly dilated ventricular system, without any dynamics in the dimensions compared to the previous study. The fourth ventricle is dilapidated and deformed. After contrasting, there is a linear dural amplification aof the signal intensity in the area of operative intervention-an expression of postoperative changes.
Conclusion
MRI data on mild internal hydrocephalus. Changes with the dural localization posterior from the 4th ventricle, possibly the expression of postoperative changes. There are no areas of shedding of contrast matter with suspicion for brain parenchyma metastases or leptomeningeal metastases (Figure 9). Two further courses of SC were conducted. In June 2013, the child was accepted for completing of the RT in the area of PCF to TD 56 Gy.
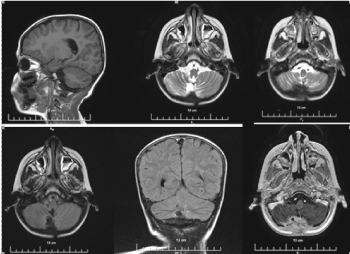
Figure 9. SAG T1, AX T2, AX T2 FLAIR and COR T2 FLAIR, 3DT1+C MR postcontrast images of the cerebrum without the presence of leptomeningeal metastases
The control MRI of the cerebrum/20.08.13.
Conclusion
MRI data on mild internal hydrocephalus. No evidence of tumour relapse or areas of suspicion for leptomeningial metastases. Treatment was discontinued and monitoring was assessed in 3-months. Three months of a control MRI of the cerebrum and spinal axis: multiple nodules with leptomeninigial localization, hyperintensity T2 and an intensity enhancing signal after contrasting are visualized. The nodules are located on the right parietal area with a diameter of 8 mm; To the right occinpitally 14 mm in diameter; Several small nodules occipital, parasagittal with a diameter of up to 4.5 mm.; To the right frontal parasagittal with a diameter of 7 mm.; In front of the upper part of the vermis, dorsal from lamina quadrigemina 11 mm in diameter. Symmetric slightly dilatable ventricular system of low- dimensional dynamics compared to the previous study. Extended and deformed IV-th ventricle. A single nodule with leptomeningeal localization is visualized along the ventral surface of the cervical myelon.
Conclusion
MRI data on multiple leptomeningial metastases, with no evidence of local relapse. Since 07.12.13, the child conducts 3 cycles of the second line of the SC with Temozolomide 120 mg/day in 5 days courses. The control MRI of the cerebrum/ 18.02.14г. - Multiple leptomeningeal metastases. From 28.02.14, the child continues with 2 more courses of SC with Vincristine, Carboplatinum and Etoposide. The control MRI of the cerebrum/23.04.14 - Without significant dynamism. In May 2014, manifests throat symptomatology as a result of increased intracranial pressure. MRI examination of the cerebrum of 03.07.14, without significant dynamics in the images compared to the previous study (Figure 10). After one month the outcome is exitus letalis.
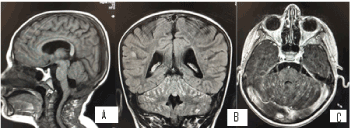
Figure 10. А/ SAG T1 МR image shows the postoperative changes in the PCF, condition after surgery and extirpation of the medulloblastoma; B/ COR T2 FLAIR after contrast МR image through IV ventricle with multiple hyperintense leptomeningeal liquid metastases, predominantly infratentorial lesions; С/ 3DT1+C МR postcontrast image with multiple contrast-enhancing leptomeningeal metastases on folia cerebelli
Clinical case № 4
Male 35 years old with complaints of headaches, misspelling, weakness of the right hand, periodic confusion and vertigo. From MRI/10.05.2011.-Subtentorial parasagittal, to the right at the level of cerebellar vermis and right cerebellar hemisphere hypointensity T1 and hyperintense focal lesion in measurements with long rehearsal times. Data for volumetric process in right cerebellar hemisphere (Figure 11).
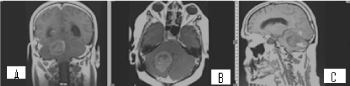
Figure 11. COR, AX and SAG 3DT1+C MR postcontrast images of the cerebrum with hypointense subtentorial focal lesion to the right at the level of cerebellar vermis and right cerebellar hemisphere, which is hyperintense in measurements with long rehearsal times
Occipital craniotomy with partial tumour extirpation has been performed. After 20 days of operation, hydrocephalus shunt valves are required. The patient is directed for reoperation to achieve maximum radical tumor extirpation with histological result undifferentiated medulloblastoma/small cell neuroblastic variant.
Immunohistochemical (IHC) analysis – positive IHC expression to NSE and negative to LCA and S-100 protein. To achieve maximal tumour extirpation, two more operations were performed, followed by CSRT to TD 36Gy with a boost in the tumour bed to TD 56 Gy. After 1.5 months from completion of CSRT, MRI without data on local relapse. After 6 courses SC under the scheme BEP, on the occasion of back pain and instability in the lower limbs, of the MRI it is established: relapse of the tumor formation in the right cerebellar hemisphere with dimensions 34/38/31 mm (transverse, anterior-posterior and cranio-caudal). Perifocal edema with compression of the 4th ventricle. Ventricular system- slightly dilated. Compression fracture of the body of vertebre Th 5. MRI data for CSF metastases (Figure 12).
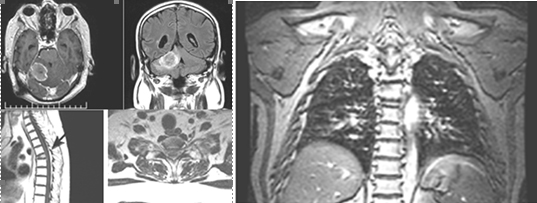
Figure 12. AX, SAG and COR 3DT1+C MR postcontrast images of neuroaxis-relapse of tumour in the right cerebellar hemisphere. Compression fracture of the body of Th 5 vertebrae and MR data for CSF metastases
A laminectomy was carried out at the level of Th5 vertebrae with partial laminectomy at the levels of Th4 and Th6 with a titanium metal stabilization of Th 4-Th 7 levels. On the occasion of hydrocephalus and dysfunction of the valvular system, decompression of the vertebral column at level Th5 and dorsal stabilization is judged. An extirpation of tumour relapse in the right cerebellar hemisphere with revision of the ventriculoperitoneal shunt device was performed. Histological result- Neuroblastic medulloblastoma. Two months after the last surgery the outcome is exitus letalis.
Clinical Case № 5
A young woman of 22 years without a family burden. In 2008, on the occasion of headaches, faltering and difficulty gait of the brain CT, the tumor formation in the PCF with pronounced hydrocephalus was established. After insertion ventriculoperitoneal shunt device complaints persist. 18.02.08 craniotomy with a visible total extirpation of the tumour formation in the PCF was performed. Histological result-medulloblastoma with spongioblastic pattern. After the operation, CSRT to TD 36Gy with a boost in the tumour bed to TD 56 Gy was performed. On the CT of the cerebrum /month 11.08, a hypotensive area on the right in the cerebellar hemisphere with a CSF density, without evidence of hydrocephalus and local relapse was reported. 5 courses of SC with BiCNU were conducted. Due to pronounced myelotoxicity, chemotherapy was interrupted and active monitoring assessed. After 9 years on the control MRI study (28.03.2017) of the cerebrum a relapse of the tumour formation with compression on the vermis and lV ventricle was established. Slightly dilated ventricular system with scars of transependial resorption. An extirpation of the tumor formation with a histological result classic medulloblastoma and 6 cycles SC with Temozolomide after surgery was performed. On the MRI (10.05.2017) data on pathological intra and extramedular lesions are lacking. MP image of the bulging disks in vertebral levels C3-С7, L1-S1. MR image of the cerebrum/18.07.2017: MR data on changes in the parenchyma of the right cerebellar hemisphere, most likely the expression of postoperative changes-edema and gliosis. A further 4 cycles of SC with Temozolomide were performed. The MRI /07.11.2017 does not take into account the MP data on the residual formation, as well as the dynamics in the images when compared with the previous 18.07.2017. The patient continues his treatment abroad. On the occasion of a relapse of 16.07.2018, a visibly total tumour extirpation followed by the SC was performed.
The MRI/12.06.2019- Persistence of right infratentorial irregular postoperative and encephalopathic cystic areas; On their background presence of a zone of pathological signal intensity in right cerebellar hemisphere, right middle cerebellar foot, a small part of vermis and left dental nuklei. This area is hyperintensе оn T2 and T2 tirm images and hypointense on T1 images. The cortical-medullary differentiation in places is erased. After contrasting, pathological amplification in the area of operative intervention are not established. Traction and deformation of lV ventricle to the right; light right dislocation of the brain stem and vermis. Several subependymal nodular areas of two lateral ventricles anterior horns with pathological signal (size up to 9/7mm) and single to the body of the left lateral ventricle (9.5 mm) are visualised. Lesions are hyperintensе оn T2 and T2 tirm images and hypointense on T1 images, diffusion restriction or postcontrast amplification is not available. High in the left cerebellar hemisphere a lesion (7.5/5mm) with similar signaling characteristics, is visualized (Figure 13). These lesions are not visualized on the MRT /23.08.2018 and on the MRT/ 13.02.2019 are poorly identified.
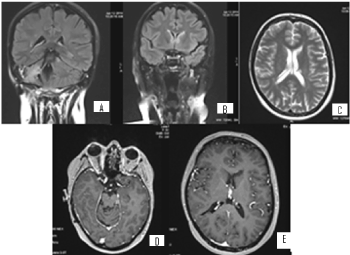
Figure 13. А/ COR T2 FLAIR after contrast МR image through IV ventricle with postoperative changes in the right cerebellar hemisphere and single hyperintense leptomeningeal lesion, peak in the left cerebellar hemisphere; В/ COR T2 FLAIR after contrast МR image of the subependimal hyperintense nodular zones, through the frontal horns bilaterally; С/ AX T2 image revealing hyperintense subependymal lesions in the frontal horns of the ventricles; D/ МR AX 3D T1+C postcontrasting images at the level of the lesions, described in the left cerebellar hemisphere; E/ МR AX 3D T1+C postcontrasting images of the level of the lesions, described through the frontal horns of the ventricles, where hypointense lesions are visible, which do not demonstrate contrast enhancement
On the MR image/ 2020, a visible progression of subependymal metastases and hypointense lesions without contrast amplification in the left cerebellar hemisphere and in the lateral ventricles were reported (Figure 14).
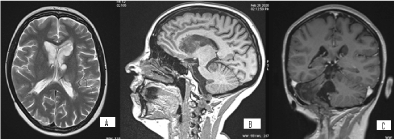
Figure 14. А/ AX T2 МR image through the lateral ventricles with multiple hyperintense subependymal masses, with a clear progression from the previous MRI; B/ SAG T1 МR image, showing the known hypointense lesions in the left cerebellar hemisphere and in the lateral ventricles, with a clear progression from the previous MRI; C/ Contrast COR T1+C МR image, which shows that the lesions are non-enhancing
Clinical case №6
We present 43 year old woman with medulloblastoma diagnosed 8 years ago/ 2012. Subtotal tumour extirpation was carried out, followed by craniospinal radiation therapy (CSRT) with TD-36 Gy and boost in cerebellum upto TD-54 Gy with daily dose (DD) 2 Gy in 27 fractions. After the operation, 6 courses of chemotherapy were conducted.
On the occasion of headaches and dizziness, on the MRI/2018, a relapse of the tumour formation in the PCF with subependimal nodules and single lesion, adjacent to the pituitary Infundubulum with the MR characteristic of CSF metastases was established (Figures 15 and 16). A slight degree of hydrocephalus is visible.
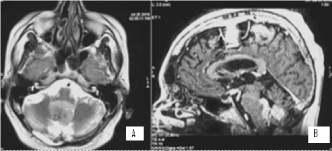
Figure 15. А/ AX T2 МR image at the level of IV ventricle indicates recurrent hyperintense tumour formation; В/ SAG 3D T1+C МR image with the recurrent contrast-enhancing tumour formation in IV ventricle and contrast-enhancing metastasis in the area of hypophyseal infundibulum
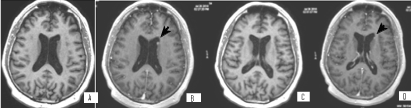
Figure 16. А и С/ Native 3D T1 МR images through the level of lateral ventricles, reporting subependimal lesions in the left lateral ventricle; В и D/ Corresponding 3D T1 + C MR postcontrasting images at the same levels, showing postcontrast enhancement of lesions
LMD remains a clinical diagnosis, based on clinical symptoms, imaging, and cerebrospinal fluid analysis (13). T1-weighted magnetic resonance imaging (MRI) with gadolinium contrast, which has been shown to be more sensitive compared to contrast enhanced CT [14,15]. All imaging should include the brain and spine, as leptomeningeal disease can impact the entire neuraxis [16].
The anatomy of the neuroaxis consists of the brain and spinal cord, covered by the meninges, which are comprised of dura mater, arachnoid membrane, and pia mater. The leptomeninges refers to the two most inner layers, arachnoid membrane and pia matter, including the subarachnoid space, which separates these two sheets, and is the location of the CSF. The CSF is the location of circulating tumor cells in patients with LMD. The pathogenesis of LMD is multifaceted, and can include direct extension from preexisting CNS tumors or systemic tumors, that follow peripheral nerves into the subarachnoid space, as well as infiltration through hematogenous dissemination, or even seeding of the subarachnoid space during surgical procedures [7,17-20]. Almost all clinically significant metastases from the medulloblastoma (MB) are located in the leptomeningeal area, clinging to the soft brain sheath under the arachnoid membrane and are poured from the CSF [21]. The metastasis model is limited to leptomeningeal space, based on assumptions and poorly maintained empirical evidence, that MB spread by direct distribution in the cerebrospinal fluid of primary tumor MB cells. Subsequently, tumor cells are implanted and grow on the surface of the soft brain matter [22]. By the presence of circulating in the blood MB tumor cells in an untreated patient, it is proved that they can spread by hematogenous way to the leptomeningial space and form LMD [21]. Recent molecular studies have shown, that MB is not a single entity but rather a constellation of groups, each with a distinct developmental origin, meaning that each may therefore benefit from subgroup-specific treatments [23-25]. On MRI, the most common finding is pial enhancement and nodularity, typically over the cerebral convexities, in the basal cisterns, on the tentorium, or in the ventricular ependymal surfaces [15,26]. Common findings on imaging of the spinal cord include patchy involvement of nerve roots with occasional matting and intradural extramedullary nodules, particularly at the cauda equina [27]. We present five clinical cases with medulloblastoma, of which one in childhood and four in adulthood. Mеduloblastoma (MB) is a malignant embryonal tumour, a subtype of primitive neuroectodermal brain tumors (PNET), predominantly growing infratentorially in the cerebellum [21]. MB is a heterogeneous disease comprising four molecular subgroups - wingless (WNT), sonic hedge hog (SHH), group 3, and group 4, with distinct developmental origins, unique transcriptional profiles, diverse phenotypes and varying clinical outcomes [24,25,28,29]. Some MR images of LMD in medulloblastoma appear to be characteristic, perhaps even specific to certain molecular subgroups [30]. This fact may help in differentiating molecular groups, in particular groups 3 and 4, when the characteristics of the primary tumour overlap [31], with potential to influence therapy [30]. The annual new incidence of MB in teenage and adulthood was only 0.05 to 100 000 [32]. Prados et al. identifies a group of patients with an adverse/bad risk including < 75% tumour extirpation, metastatic disease and brainstem invasion or LMD [33].
Discussion of the characteristics of the presented clinical cases with LMD:
Clinical case №1 is an adulthood classic type medulloblastoma in PCF, after surgery and RT. After 4 years and disease progression-free survival, three local relapses in the cerebellum had been manifested, which had been surgically removed. Аfter the third reoperation, systemic chemotherapy (SC) was performed. On the МRI, diffuse subependymal periventriculare metastases, combined with leptomeningeal metastases in the cerebellum and the entire spinal axis, showing postcontrast enhancement, were established. Three months after the completion of the re-CSRT, we conducted an MRI of the brain and cervical spinal cord, which reported a lack of subependimal and leptomeningeal metastases in the brain, cerebellum and cervical spinal axis. This result confirms the strong radiesensitivity of the leptomeningeal metastatic meduloblastoma cells. It is necessary to continue the complex treatment with SC and target therapy. In clinical case №2 develops leptomeningeal metastatic disease (LMD) after 10 years of diagnosis and the complex treatment of invasive intraductal carcinoma of the right mammary gland/pT2N0M0, G2, positive estrogen and progesterone receptors, HER2/+++/. The disease progresses with hematogenous metastasis in the lung and liver, despite repeated courses of SC and targeted therapy. Patients with cerebral involvement typically receive whole brain radiotherapy (WBRT), which is planned to involve all neural tissue from the retro-orbit to the upper cervical vertebrae (16). In this case, consider late LMD with a typical MR image of postcontrast enhancement. In clinical case №3 consider late diagnostics, 5 months after a gradual worsening symptomatology. Despite the more favourable prognosis in non-metastatic desmoplastic medulloblastoma in childhood, five months are sufficient for metastatic disease on the leptomeningeal and CSF pathway. After complex therapy, including surgery, SC and CSRT with a boost in the tumour bed up to 56 Gy, local tumor control (LTC) of metastatic disease was achieved. Regardless of systemic and local control of the primary disease, prognosis in the setting of LMD is very poor, with reported average survivals of ~2–4 months, even with treatment [34,35]. As a result of the complex treatment, 2 years of overall survival was reported. Clinical case №4 is an extremely aggressive histological variant of medulloblastoma (undifferentiated, small-cell neuroblastic) adulthood. It is at high risk of local relapses and leptomeningial metastases. 6 months after the complex treatment, including multiple operations, CSRT and 6 courses SC regimen scheme BEP, consider extremely unfavorable development of the disease with local relapse and CSF metastases. As a result of the complex treatment consider 1.5 years overall survival with disease progression. To improve the healing results in adulthood type medulloblastoma with similar histology, radical surgery, hyperfractionated CSRT, SC and target therapy are offered. Clinical case № 5 is medulloblastoma with spongioblastic pattern at a young age after complex treatment. After 11 years, a local relapse was manifests, which surgically with a subsequent SC regimen/ scheme Temodal, was treated. After 2 years with progression over time, a subependimal leptomeningeal metastases develops with a cerebellar-CSF metastasis on the left parsagitally. The special in this case is the lack of contrast enhancement („mismatching” pattern) of the leptomeningeal metastases on the MRI. Similar examples have been found in the literature, where it is demonstrated, that the absorption of the contrast depends on the genetic type and the histological variant of the meduloblastoma cells [28,30,31]. This fact increases the significance of the MRI, not only for the diagnosis and staging of meduloblastoma, but also for the preliminary determination of the prognosis, closely related to the complex treatment. Clinical case № 6 is similar to case No1- Medulloblastoma in adulthood after complex treatment, including multiple surgeries, CSRT, 6 courses SC. After 6 years, the manifestation of local relapse with subependimal leptomeningeal metastases and typical contrast enhancement of MRI.
1. Brain and spinal cord MRI is useful and necessary not only for the diagnosis and staging of meduloblastoma, but also for predicting MB molecular subgroups, which is directly related to the prognosis and necessary complex treatment.
2. Brain and spinal cord MRI is required in the diagnosis of late LMD in the case of extracranial solid neoplasms. Radiation therapy has a different target volume and necessary radiation doses, depending on the histological appearance of tumor cells, as well as on the volume and localization of leptomeningeal metastases.
- Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, et al. Harrison's Principles of Internal Medicine. 17th ed. New York, NY: McGraw Hill (2008).
- Nugent JL, Bunn PA Jr, Matthews MJ, Ihde DC, Cohen MH, et al. (1979) CNS metastases in small cell bronchogenic carcinoma: increasing frequency and changing pattern with lengthening survival. Cancer 44: 1885-1893. [Crossref]
- Shapiro WR, Posner JB, Ushio Y, Chemik NL, Young DF (1977) Treatment of meningeal neoplasms. Cancer Treat Rep 61: 733-743. [Crossref]
- Youmans JR, Winn HR. Youmans neurological surgery. Philadelphia, PA: Saunders/Elsevier; 2011.
- Clarke JL, Perez HR, Jacks LM, Panageas KS, Deangelis LM (2010) Leptomeningeal metastases in the MRI era. Neurology 74: 1449-1454. [Crossref]
- Hyun JW, Jeong IH, Joung A, Cho HJ, Kim SH, Kim HJ (2016) Leptomeningeal metastasis: Clinical experience of 519 cases. Eur J Cancer 56:107.
- Aroney RS, Dalley DN, Chan WK, Bell DR, Levi JA (1981) Meningeal carcinomatosis in small cell carcinoma of the lung. Am J Med 71: 26-32. [Crossref]
- Glass JP, Melamed M, Chernik NL, Posner JB (1979) Malignant cells in cerebrospinal fluid (CSF): the meaning of a positive CSF cytology. Neurology 29: 1369-1375. [Crossref]
- Andrew L Wagner and James G Smirniotopoulos. Leptomeningeal Carcinomatosis (Metastasis) Imaging Medscape; Drug$ Disease Updated: Jul 10, 2019.
- Costa R, Kumthekar P: Management of central nervous system metastases in breast cancer. The Breast (Fifth Edition). Bland KI, Copeland EM, Klimberg VS, Gradishar WJ (ed): Elsevier, Amsterdam; 2018. 942-960.
- An YJ, Cho HR, Kim TM (2015) An NMR metabolomics approach for the diagnosis of leptomeningeal carcinomatosis in lung adenocarcinoma cancer patients. Int J Cancer 136: 162-171.
- Chamberlain MC (2013) Comprehensive neuraxis imaging in leptomeningeal metastases: a retrospective case series. CNS Oncol 2:121-128.
- Harris P, Diouf A, Guilbert F (2019) Diagnostic Reliability of Leptomeningeal Disease Using Magnetic Resonance Imaging. Cureus 11(4): e4416.
- Chamberlain MC, Sandy AD, Press GA (1990) Leptomeningeal metastasis: a comparison of gadolinium-enhanced MR and contrast-enhanced CT of the brain. Neurology 40: 435-438. [Crossref]
- Collie DA, Brush JP, Lammie GA, Grant R, Kunkler I, et al. (1999) Imaging features of leptomeningeal metastases. Clin Radiol 54: 765-771. [Crossref]
- Gautam Nayar, Tiffany Ejikeme, Pakawat Chongsathidkiet (2017) Leptomeningeal disease: current diagnostic and therapeutic strategies. Oncotarget 8(42): 7331.
- Price RA, Johnson WW (1973) The central nervous system in childhood leukemia. I. The arachnoid. Cancer 31: 520-533. [Crossref]
- Rosen ST, Aisner J, Makuch RW, Matthews MJ, Ihde DC, et al. (1982) Carcinomatous leptomeningitis in small cell lung cancer: a clinicopathologic review of the National Cancer Institute experience. Medicine (Baltimore) 61: 45-53. [Crossref]
- Vannier A, Gray F, Gherardi R, Marsault C, Degos JD, et al. (1986) Diffuse subependymal periventricular metastases. Report of three cases. Cancer 58(12): 2720-5.
- Spiegel S, Pelz DM, Fox AJ, Vinuela F (1985) Subependymal metastases of an extracranial malignancy. J Can Assoc Radiol 36: 334-336. [Crossref]
- Nieder C, Grosu AL, Andratschke NH, Molls M (2005) Proposal of human spinal cord reirradiation dose based on collection of data from 40 patients. Int J Radiat Oncol Biol Phys 61: 851-855. [Crossref]
- Al-Anazi A, Shannon P, Guha A (2000) Solitary metastasis to the choroid plexus. Case illustration. J Neurosurg 92: 506. [Crossref]
- Cavalli FM, Remke M, Rampasek L (2017) Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell 31.
- Ramaswamy V, Remke M, Adamski J (2016) Medulloblastoma subgroup-specific outcomes in irradiated children: who are the true high-risk patients? Neuro Oncol 18:291.
- 25.Thompson EM, Hielscher T, Bouffet E (2016) Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated clinical and molecular analysis. Lancet Oncol 17: 484.
- Gleissner B, Chamberlain MC (20016) Neoplastic meningitis. Lancet Neurol 5: 443.
- Freilich RJ, Krol G, DeAngelis LM (1995) Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastasis. Ann Neurol 38: 517.
- Dasgupta A, Gupta T (2018) Radiogenomics of medulloblastoma: imaging surrogates of molecular biology. J Transl Genet Genom 2: 15.
- Gajjar A, Chintagumpala M, Ashley D (2006) Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stemcell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol 7: 813.
- Colafati GS, Voicu IP, Carducci C, Miele E, Carai A, et al. (2018) MRI features as a helpful tool to predict themolecular subgroups of medulloblastoma: state of the art. Ther Adv Neurol Disord 11: 1756286418775375.
- D. Mata-Mbemba, M. Zapotocky, S. Laughlin (2018) MRI Characteristics of Primary Tumors and Metastatic Lesions in Molecular Subgroups of Pediatric Medulloblastoma: A Single-Center Study. AJNR Am J Neuroradiol.
- Veninga T, Langendijk HA, Slotman BJ, Rutten EH, van der Kogel AJ, et al. (2001) Reirradiation of primary brain tumours: survival, clinical response and prognostic factors. Radiother Oncol 59: 127-137. [Crossref]
- Prados MD, Warnick RE, Wara WM, Larson DA, Lamborn K, et al. (1995) Medulloblastoma in adults. Int J Radiat Oncol Biol Phys 32: 1145-1152. [Crossref]
- Hitchins RN, Bell DR, Woods RL, Levi JA (1987) A prospective randomized trial of single-agent versus combination chemotherapy in meningeal carcinomatosis. J Clin Oncol 5: 1655-1662. [Crossref]
- Waki F, Ando M, Takashima A, Yonemori K, Nokihara H, et al. (2009) Prognostic factors and clinical outcomes in patients with leptomeningeal metastasis from solid tumors. J Neurooncol 93: 205-212. [Crossref]
















