Abstract
Bile acids are important and necessary for the effective lipid digestion. Recently, bile acid receptors have been detected at different tissues such as liver, placenta, lung, spleen, breast, adrenal gland, pituitary gland, kidney, brain and skeletal muscle. TGR5 and FXR are the mostly known bile acid receptors in many tissues. Bile acids can effect both carbohydrate and lipid metabolism, and intrauterine growth by stimulating the bile acid receptors. Bile acid receptors, especially the TGR5 and FXR may have important roles on lots of metabolisms. Bile acid levels may be suggested in the etiopathogenesis of unexplained metabolic disorders. Future studies particularly regarding the receptor-associated gene expression might produce new investigations for many metabolic disease therapies. Bile acid receptor-based animal studies have shown that bile acid receptor agonists or antagonists can be used in the treatment of many diseases. Bile acid receptors such as TGR5 and FXR may also be used as diagnostic markers in some diseases. In addition, gut microbiota contributes to the physiological and pathological activity of bile acids and salts by increasing the diversity, and may play decisive and important roles in bile acid metabolism.
Key words
Bile acids, FXR, TGR5
Introduction
Bile acids, 24-carbon steroids synthesized from cholesterol in the liver, are concentrated and stored in the gall bladder, then released into the duodenum for the digestion of fatty foods, especially after diet. Cholesterol conversion into primary bile acids, cholic acid (CA) and chenodeoxycholic acid (CDCA), occurs via two different pathways; the classical pathway (more than 75% of total bile acid pool) and the alternative pathway (less than 25% of total bile acid pool). These primary bile acids occur in the liver by the addition of hydroxyl groups to the 7thor 27thcarbon atom of cholesterol, modification of the sterol ring, oxidation and shortening of the side chain, and conjugation. In this reaction chain, the rate-limiting enzyme is cholesterol 7-α hydroxylase (CYP7A1). Cholesterol 7-hydroxylase (CYP7A1), sterol-27 hydroxylase (CYP27A1), sterol 12-hydroxylase (CYP8B1) are the major regulatory enzymes in the synthesis of primary bile acids. These primary bile acids are partially re-absorbed in the small intestine, and the portion that is not absorbed is deconjugated and metabolized by the gut flora of the intestine, especially the colon, to the secondary bile acids of deoxycholic acid and lithocholic acid (Figure 1). Conjugation of bile acids with glycine and taurine in the liver results with bile salts, which increase the solubility of bile acids in physiological fluids. The bile salts have a stronger detergent effect than the bile acids due to the strengthened amphipatic structure resulting from the conjugation. The role of intestinal bacteria in the conversion of bile acids to each other is known. However, the influence of intestinal microbiota on bile acid and bile salt diversity has become an important and controversial subject [1,2].
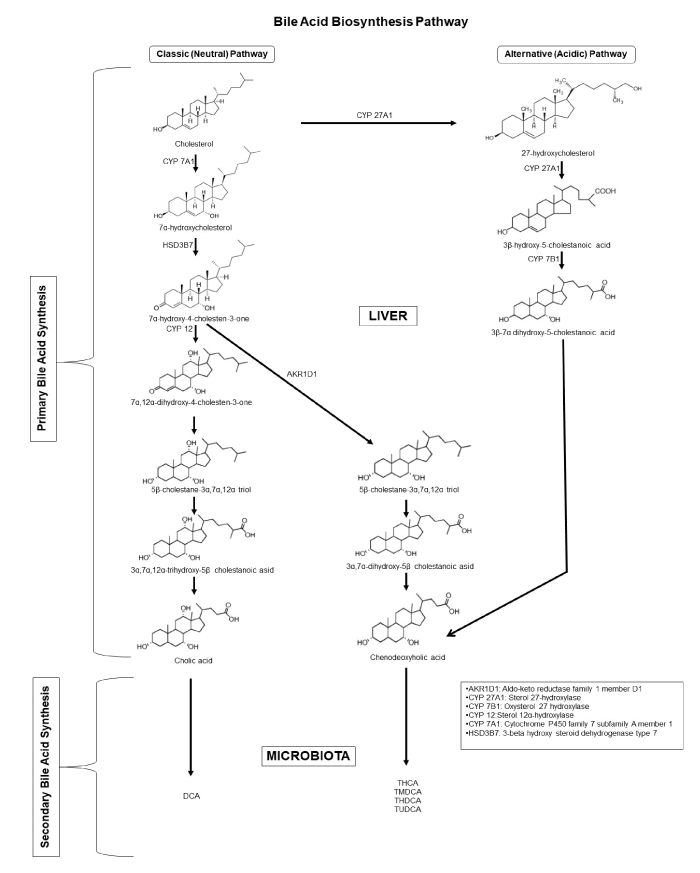
Figure 1. Bile acid synthesis.
Bile acid receptors
It has recently been determined that bile acids have many metabolic effects besides their digestive function. Endocrine and paracrine effects of some receptors activated by bile acids have been determined [3]. It has been shown that Farnesoid X Receptor (FXR), Pregnan X Receptor (PXR), Constitutive Androstane Receptor (CAR), and Vitamin D Nuclear Receptor (VDR) are stimulated by bile acids [4]. In addition, the membrane receptor G-Protein Coupled Receptor (GPCR), a member of the Takeda G-Protein-Coupled Receptor 5 (TGR5) group, is also stimulated by the bile acids [5]. These receptors are detected in the liver, placenta, lung, spleen, breast, adrenal gland, kidney, pituitary gland, and skeletal muscle [6,7]. Bile acids show their effects in carbohydrate and lipid metabolism, cell proliferation, liver regeneration, cell apoptosis, homeostasis, energy metabolism and heat modulation through these signalling molecules [8].
Bile acids in carbohydrate metabolism
Bile acids show effects on carbohydrate metabolism by stimulating FXR and TGR5 receptors [9]. Stimulation of Glucagon Like Peptide 1 (GLP 1) released from incretin peptides as a result of TGR5 receptor stimulation in intestinal L-cells may be shown as a concrete example of the relationship between bile acids and diabetes. GLP1 is required to improve blood sugar by stimulating insulin secretion and suppressing the release of glucagon [10]. FXR expression in pancreatic β-cells is important for the effect of bile acids on glucose metabolism [11]. It has been shown that insulin resistance develops in FXR knockout mice, while glucose levels decrease in obese and diabetic mice models taking FXR agonist therapy. Moreover, increased gluconeogenesis and lipogenesis were found in FXR deficient mice. In diabetic mice models, when FXR agonist was given, hepatic glycogen synthesis was increased and glyconeogenesis was suppressed. These studies showed the role of the FXR receptor in carbohydrate metabolism [12,13]. It was also found that obesity did not develop, insulin resistance decreased, and energy expenditure increased in mice fed with diets supplemented with bile acids [14].
Bile acids in lipid metabolism
Bile acids effect both digestion and regulation in the lipid metabolism via FXR receptor [15]. A proatherogenic lipid profile of significantly elevated serum cholesterol and triglyceride levels was observed in FXR knockout mice. Bile acids reduce FXR-dependent triglyceride levels and lipogenesis [16]. In addition, activated FXR is directly or indirectly involved in the transcription of many genes that play a role in the fatty acid and triglyceride synthesis. Steroid Regulatory Element Binding Protein 1-C stimulated by active FXR receptor is effective in regulating the lipid metabolism. However, hepatic FXR activation regulates CYP7A1 in negative direction [1]. In cardiovascular diseases, bile acids may be important due to their antiatherogenic function. The vasodilator effect of the TGR5 and FXR receptors of may be important in cardiovascular system regulation due to the effectiveness of bile acids on the regulation of lipid metabolism, and inhibition of the atheromatous plaque formation. Furthermore, bile acid receptors on myocytes may affect the myocardial transmission and contractility [17]. It has been shown that bile sequestering agents not only reduce the LDL cholesterol and triglyceride levels, but also reduce the risk of cardiovascular disease development, and improve the glycemic control in type 2 diabetic patients [18].
Bile acids in liver metabolism
TGR5 is detected in kupffer cells, cholangiocytes and sinusoidal endothelial cells. TGR5 activation is important for the intracellular cAMP elevation and intracellular signal conduction. TGR5 has anti-inflammatory effects, protects cholangiocytes from toxicity, reduces portal perfusion pressure, and promotes cholangiocytes proliferation and secretion. Proliferative and regenerative capacities of liver reduce in the lack of TGR5 receptor stimulation. It has been shown in animal studies that TGR5 receptor stimulation protects against to some pathologies such as steatohepatitis and liver fibrosis, but TGR5 activation may increase the risk of polycystic liver diseases and cholangiocarcinoma [19-25]. TGR5 antagonist agents therefore may have an important role in treatment of these pathologies. As a result, both TGR5 receptor activation and inhibition may have important role in different liver disease treatments [26]. The positive effect of bile acids on liver regeneration can also be assessed in terms of FXR. Many animal studies have shown that intrahepatic levels of bile acids increase rapidly after resection. It has been reported that FXR, activated by bile acids, regulates hepatic metabolism and promotes hepatocellular proliferation. It has also been explained that effect of FXR in liver regeneration happens by stimulating the Fibroblast Growth Factor (FGF) 15/19 gene expression. Animal studies have demonstrated the necessity of FXR for liver regeneration after partial hepatectomy and acute toxic liver injury. The clarification of the relationship between FXR and FGF 15/19 may result with the development of hepatoprotective and proregenerative therapeutic strategies in acute and chronic liver injuries [27].
Bile acids in renal metabolism
FXR has been shown to induce proliferation of renal adenocarcinoma cells. In a mouse study, renal adenocarcinoma cell growing was suppressed by FXR knockdown without affecting normal renal cells. The contribution of FXR to adenocarcinoma pathogenesis has been explained by the downregulation of tumour suppressor gene p53 and DNA repair gene p21/cip 1 mRNA [28]. Bile acid receptor studies may be useful not only for explaining the pathogenesis of renal cancers, but also for finding diagnostic markers and therapeutic agents. In another experimental animal study, the effects of bile acid receptors, FXR and TGR5, on aging and caloric restriction were investigated in the kidney. It was found that FXR and TGR5 bile acid receptors expression levels decrease with renal aging, and caloric restriction blocks this dysfunction. In this study, 22 months old ames type dwarf mice were treated with the FXR-TGR5 dual agonist INT-767 for 2 months and age-related renal disease was reduced in these mice. After the treatment, similar effects of caloric restriction were detected in the kidneys. The findings of proteinuria, podocyte damage, fibronectin accumulation, TGF-β expression and mitochondrial function in mice were also detected inversely with age. In addition, it was detected that INT-767 inhibits increase of proinflammatory markers such as TNF-α, Toll-Like Receptor (TLR)-2, TLR-4 in old mice podocytes. Bile acid receptors of TGR5 and FXR may play an important role in the regulation of age-related renal disease [29]. FXR and TGR5 studies may also be useful for explaining the nephropathy pathogenesis and developing the diagnostic markers and therapeutic agents.
Bile acids in bone metabolism
FXR expression was detected in calvaria and bone marrow cells. FXR +/+ male mice were compared with FXR -/- male mice according to the osteoblastic and osteoclastic activities. Bone mineral density was detected 4.3-6.6% lower in FXR -/- mice following 8-20 weeks. FXR deficiency resulted with the decrease in bone formation rate, trabecular bone volume and thickness. Increased osteoclastic activity was also detected in mice with FXR deficiency. It has been shown that FXR agonists and FXR activation increase osteoblastic differentiation, and suppress osteoclastic differentiation in the bone marrow. These results demonstrate the importance and function of FXR in bone metabolism [24]. Bile acid receptor studies thus may be important and hopeful for the bone disease therapies since FXR effects the osteoblastic and osteoclastic activities. In addition, long term chenodeoxycholic acid treatment was shown to increase the bone mineral density in Cerebrotendinous Xanthomatosis patients with low bone density [25].
Bile acids in brain function
TGR5, expressed in neurons and astrocytes, has been stimulated by pregnan and allopregnanolone as a neurosteroid receptor in brain tissue [22]. In an experimental study, the effect of TGR5 on hepatic encephalopathy was demonstrated. The male mice were infused with TGR5 agonist betulinic acid intracerebroventriculary for three days, and then were given azoxymethane intraperitoneally to achieve acute liver failure. It was seen that in this hepatic encephalopathy mouse model the time to reach the coma was longer in the TGR5 agonist given group. It was observed that signal transmission occurred, and neurological deficit formation decreased in the betulinic acid given group. After betulinic acid treatment microglia cells and neurons decreased chemokine release of CCL2, reduced proinflammatory cytokine production, and decreased phagocytic activity. These results demonstrate the neuroprotective effect of TGR5 agonist on the hepatic encephalopathy pathogenesis [23]. Therefore, antiphagocytic effect of TGR5 expression may have important consequences in the treatment of neurodegenerative diseases.
Bile acids in lung cancers
It was investigated whether FXR bile acid receptor is an oncogene or not. The results of this study showed that FXR significantly increased in non-small cell lung cancer (NSCLC). It was seen that, in vitro cell proliferation in NSCLC cells were inhibited by FXR knockdown, but stimulated by FXR overexpression. The tumor suppressor role of FXR knockdown in cancer pathogenesis is to delay G1/S transition in cell cycle, thus uncontrolled cell proliferation is prevented. It has been shown that FXR prevented controlled cell proliferation by increasing the transcription of cyclin D1 which is responsible for controlled cell proliferation in G1/S transition. It was detected that FXR and Cyclin D1 in patients with NSCLC were significantly higher than normal group, and these two molecules showed positive correlation. In addition, patients with over expression of both FXR and cyclin D1 had worse prognosis than the other patients [30]. These results demonstrate that FXR may be an effective oncogene in the pathogenesis of NSCLC, may have an important role in the controlled cell proliferation, and may be useful for the prognostic and therapeutic purposes.
Bile acids in intrauterin process
In the placenta, bile acid-sensitive VDR, FXR and PXR receptors were detected. These receptors interact with the bile acids and may play an important role in nutrient transport, mineral metabolism, cell proliferation and differentiation. In addition, the other known receptor, TGR5, was stimulated by bile acids, and was detected in the human and mouse placenta. TGR5 expression in human placenta was found lower in patients with intrahepatic cholestasis of pregnancy than in the control group [19]. The progesterone and progesterone metabolites released from the placenta after eight weeks of the gestation were found as the FXR and TGR5 agonists. Progesterone released from the placenta is thought to be effective with TGR5-associated cholestasis [20,21]. FXR and TGR5 receptors may be considered as the potential therapeutic targets in in patients with intrahepatic cholestasis of pregnancy.
Gut Microbiota and bile acids
The relationship between gut microbiota and bile acid metabolism has recently been an important debate issue. Because, bile acids are thought to encounter with gut microbial enzymes during the enterohepatic circulation, and bile acid diversity increases as a result of this interaction. It is known that the conversion of primary bile acids, cholic acid and chenodeoxycholic acid, to secondary bile acids, deoxycholic acid and lithocholic acid, happens by gut bacteria. However, considering the increasing number of defined bile acids today, this subject is thought to be much more complex. Gut microbiota is thought to contribute significantly to the bile acid diversity in the bile acid pool, since gut microbial enzymes are effective in deconjugation and dehydroxylation reactions for the production of secondary bile acids and unconjugated bile acids. Although microbiota is important in the bile acid regulation, antibiotic use, age, dietary and disease states may cause dysregulation of this collaboration. Microbial enzyme changes can also affect the bile acid production, so that bile acid and receptor interaction can change. These interaction changes can lead to different physiological consequences in the body [31,32]. The interactions in microbiota may primarily be important in the production and diversity of bile acids.
Conclusion
Bile acids show hormone-like effects, particularly by stimulating the nuclear receptor FXR and the membrane receptor TGR5. Bile acids are effective in digestion and have hormonal effects in carbohydrate and lipid metabolisms by specifically stimulating the receptors of FXR and TGR5. Furthermore, these receptors detected in liver, lung, brain, bone, kidney, intestinal tract and placenta have tissue specific role, and give signals that bile acids can affect the entire endocrine system. The role of bile acids in the treatment of diseases related to carbohydrate and lipid metabolism disorders can be investigated by multicentre studies. In addition, bile acid levels may be suggested in the etiopathogenesis of unexplained metabolic diseases. Since there are bile acid receptors on lots of systems, receptor-associated bile acid studies may elucidate the diagnosis and treatment of many metabolic diseases. In addition, the efficacy of treatment can be determined by testing the different drugs acting on bile acid receptors. Future studies particularly regarding the receptor-associated gene expression may also produce new insights in improving medical therapies. Currently positive results have been obtained in the experimental animal studies of bile acid agonist and antagonist agents on. Especially the new bile acid receptor agonist and antagonist agent investigations may contribute to the treatment of many metabolic disease in the future. In addition, bile acid receptors may be used as a biomarker in the diagnosis of many benign and malign diseases. Positive results for diagnostic purposes have been observed in studies evaluating FXR expression on different types of cancer. Gut microbiota which increases the diversity of bile acids and salts may be decisive and effective factor in the bile acid metabolism. The contribution of gut microbiota to bile acid metabolism may be important for the physiological effect of bile acid on different organs. Gut microbiota which is a direct contributor to the bile acid diversity must absolutely be considered when examining and understanding bile acid metabolism and its effects on different systems. The identification of the interaction between bile acids and gut microbiota, will give different perspective to the scientific investigations.
Highlights
The strengths of this article are as following: First, it explains most of the metabolisms through bile acids and bile acid receptors. This article shows the presence of bile acid receptors in lots of tissues with physiological or pathological mechanisms. Second, the present article has molecular and genetic approaches for the bile acid receptors, and their effects on different metabolisms. Third, discussion of the relationship between bile acids and microbiota may be an important issue for understanding the mechanisms of unexplained metabolic disorders that may be effective for the development of new therapeutic strategies.
The weaknesses of this article are firstly it does not mention the bile acid assay methods, reference intervals, and whether it has circadian rhythm or not. Secondly, the article does not deal with the factors that influence the bile acids level in the body. Thirdly, the relationship between bile acid levels and nutrition and synthesis has not been explained.
References
- Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K (2008) Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov 7: 678-693. [Crossref]
- Onur ND, Beyler AR (2001) Safra Asitleri Metabolizmasi. Ankara Üniversitesi Tip Fakültesi Mecmuasi 54: 65-76.
- JP Arab, Karpen SJ, Dawson PA, Arrese M, Trauner M (2017) Bile Acids and Nonalcoholic Fatty Liver Disease: Molecular Insights and Therapeutic Perspectives. Hepatology 65: 350-362. [Crossref]
- Li T, Chiang JY (2014) Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev 66: 948-983. [Crossref]
- Jourdainne V, Péan N, Doignon I, Humbert L, Rainteau D, et al. (2015) The Bile Acid Receptor TGR5 and Liver Regeneration. Dig Dis 33: 319-326. [Crossref]
- Duboc H, Taché Y, Hofmann AF (2014) The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig Liver Dis 46: 302-312. [Crossref]
- Ding L, Yang L, Wang Z, Huang W (2015) Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm Sin B 5: 135-144. [Crossref]
- Chai J, Zou L, Li X, Han D, Wang S, et al. (2015) Mechanism of bile acid-regulated glucose and lipid metabolismin duodenal-jejunal bypass. Int J Clin Exp Pathol 8: 15778-15785. [Crossref]
- Trabelsi MS, Lestavel S, Staels B, Corret X (2016) Intestinalbile acid receptors are key regulators of glucose homeostasis. Proc Nutr Soc 16: 1-11. [Crossref]
- Sonne DP, Hansen M, Knop FK (2014) Bile acid sequestrants in type 2 diabetes: potential effects on GLP1 secretion. Eur J Endocrinol 171: R47-65. [Crossref]
- Shihabudeen MS, Roy D, James J, Thirumurugan K (2015) Chenodeoxycholic acid, an endogenous FXR ligand alters adipokines and reverses insulin resistance. Mol Cell Endocrinol 414: 19-28. [Crossref]
2021 Copyright OAT. All rights reserv
- Ma K, Saha PK, Chan L, Moore DD (2006) Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest 116: 1102-1109. [Crossref]
- Stayrook KR, Bramlett KS, Savkur RS, Ficorilli J, Cook T, et al. (2005) Regulation of carbohydrate metabolism by the farnesoid X receptor. Endocrinology 146: 984-991. [Crossref]
- Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, et al. (2006) Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439: 484-489. [Crossref]
- Schmitt J, Kong B, Stieger B, Tschopp O, Schultze SM, et al. (2015) Protective effects of farnesoid X receptor (FXR) on hepatic lipid accumulation are mediated by hepatic FXR and independent of intestinal FGF15 signal. Liver Int 35: 1133-1144. [Crossref]
- Lambert G, Amar MJ, Guo G, Brewer HB Jr, Gonzalez FJ, et al. (2003) The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J Biol Chem 278: 2563-2570. [Crossref]
- Khurana S, Raufman JP, Pallone TL (2011) Bile acids regulate cardiovascular function. Clin Transl Sci 4: 210-218. [Crossref]
- Brufau G, Stellaard F, Prado K, Bloks VW, Jonkers E, et al. (2010) Improved glycemic control with colesevelam treatment in patients with type 2 diabetes is not directly associated with changes in bile acid metabolism. Hepatology 52: 1455-1464.
- Keitel V, Spomer L, Marin JJ, Williamson C, Geenes V, et al. (2013) Effect of maternal cholestasis on TGR5 expression in human and rat placenta at term. Placenta 34: 810-816. [Crossref]
- Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, et al. (2002) Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun 298: 714-719. [Crossref]
- Sato H, Macchiarulo A, Thomas C, Gioiello A, Une M, et al. (2008) Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies. J Med Chem 51: 1831-1841. [Crossref]
- Keitel V, Görg B, Bidmon HJ, Zemtsova I, Spomer L, et al. (2010) The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia 58: 1794-1805. [Crossref]
- McMillin M, Frampton G, Tobin R, Dusio G, Smith J, et al. (2015) TGR5 signaling reduces neuroinflammation during hepatic encephalopathy. J Neurochem 135: 565-576. [Crossref]
- Cho SW, An JH, Park H, Yang JY, Choi HJ, et al. (2013) Positive regulation of osteogenesis by bile acid through FXR. J Bone Miner Res 28: 2109-2121. [Crossref]
- Martini G, Mignarri A, Ruvio M, Valenti R, Franci B, et al. (2013) Long-term bone density evaluation in cerebrotendinous xanthomatosis: evidence of improvement after chenodeoxycholic acid treatment. Calcif Tissue Int 92: 282-286. [Crossref]
- Reich M, Klindt C, Deutschmann K, Spomer L, Häussinger D, et al. (2017) Role of the G Protein-Coupled Bile Acid Receptor TGR5 in Liver Damage. Dig Dis 35: 235-240. [Crossref]
- Alvarez-Sola G, Uriarte I, Latasa MU, Jimenez M, Barcena-Varela M, et al. (2017) Bile acids, FGF15/19 and liver regeneration: From mechanisms to clinical applications. Biochim Biophys Acta. [Crossref]
- Fujino T, Sakamaki R, Ito H, Furusato Y, Sakamoto N, et al. (2017) Farnesoid X receptor regulates the growth of renal adenocarcinoma cells without affecting that of a normal renal cell-derived cell line. J Toxicol Sci 42: 259-265. [Crossref]
- Wang XX, Luo Y, Wang D, Adorini L, Pruzanski M, et al. (2017) A dual agonist of farnesoid X receptor (FXR) and the G protein-coupled receptor TGR5, INT-767, reverses age-related kidney disease in mice. J Biol Chem 292: 12018-12024. [Crossref]
- You W, Chen B, Liu X, Xue S, Qin H, et al. (2017) Farnesoid X receptor, a novel proto-oncogene in non-small cell lung cancer, promotes tumor growth via directly transactivating CCND1. Sci Rep 7: 591. [Crossref]
- Long SL, Gahan CGM, Joyce SA (2017) Interactions between gut bacteria and bile in health and disease. Mol Aspects Med 56: 54-65. [Crossref]
- Joyce SA, Gahan CG (2017) Disease-Associated Changes in Bile Acid Profiles and Links to Altered Gut Microbiota. Dig Dis 35:169-177. [Crossref]

