Objectives: Numerous antenatal ultrasound criteria have been described to determine the prognosis of omphalocele. However, the resulting prenatal counseling often appears too pejorative. The aim of this study was to assess in a large cohort whether mortality and adverse neonatal outcomes could be related to the early prenatally observed abdominal content of omphalocele.
Methods: We conducted a retrospective cohort study at Necker Enfants Malades Hospital between 2008 and 2018. We chose to review ultrasound data before the 15th week of amenorrhea (WA, and between 20 and 28 WA. Ultrasound data collected included only the abdominal content of omphalocele. Abdominal content of omphalocele was dispatched in four groups: bowel, liver only, liver and bowel, and liver+ bowel and stomach.
Results: Seventy five patients with antenatal diagnosis of omphalocele in the first trimester were included. Overall mortality was 10% (n=8). After exclusion of dead patients, neonatal high morbidity according to our composite criterion occurred in 15,2% (n=11/72). Before 15 WA, when abdominal content was bowel and liver, the mortality observed was 23% and morbidity was 28.5%. When abdominal content was bowel, liver and stomach, the mortality observed was 20% and morbidity was 60%. Between 20 and 28 WA, when abdominal content was bowel and liver, the mortality observed was 12.5% and morbidity was 25%. When abdominal content was bowel, liver and stomach, the mortality observed was 50% and morbidity was 50%. In the second trimester (20-28 WA), omphalocele containing stomach was significantly associated with mortality (62,5% (n=5) vs 7,5% (n=5) p < 0,001) and morbidity (45,5% (n=5) vs 0%, p < 0,01).
Conclusion: Abdominal content of omphalocele seems to be useful to guide early prenatal counseling. It is an ultrasound variable easily reproduced but its interpretation may change along pregnancy. The presence of stomach in omphalocele is associated with mortality and high morbidity. This factor is thus of great prognostic value, especially when observed in the first trimester because the pejorative outcome can be early outlined. The presence of liver and bowel in the omphalocele during the first trimester is better than liver only as predicting factor of morbidity. However, abdominal content in these cases is more likely to change along pregnancy, shifting either to more or less pejorative.
omphalocele, ultrasound variables, outcome, prenatal counselling
Omphalocele is a defective closure of the anterior abdominal wall that results in herniation of abdominal viscera within the umbilical cord. The size of the abdominal defect is quite variable, ranging from minor bowel hernia to almost complete abdominal protrusion. This malformation has an overall mortality rate as high as 17 to 41% [1]. Marshall et Al reported a mortality rate of 28,7% in United States, 75% of which affects babies in the first 28 days of life [2].
Prenatal diagnosis is usually made in the first trimester and having relevant prognostic markers at such an early stage would be highly desirable. The prognosis of infants with omphalocele has been linked to many factors such as size, integrity of peritoneum and amnion, birth term, birth weight, associated anomalies and methods of closure [3]. Numerous antenatal ultrasound criteria have thus been described to determine the prognosis [4-8]. However, the resulting prenatal counseling often appears too pejorative [9]. Moreover, many authors consider difficult surgical closure as a morbidity main factor, while this criterion does not really influence neonatal outcome [10].
The aim of this study was to assess in a large cohort whether mortality and adverse neonatal outcomes could be related to the early prenatally observed abdominal content of omphalocele.
We conducted a retrospective cohort study of patients prenatally referred for omphalocele at Necker-Enfants Malades (NEM) Hospital between 2008 and 2018. Patients were included when sonographic diagnosis of omphalocele was identified before 15 WA, for babies born and postnatally evaluated in Necker-Enfants Malades Hospital. We excluded cases of termination of pregnancy, in utero fetal demise, neonatal demise with karyotype abnormality, misdiagnosis, sonographic diagnosis after 15 WA and patients lost to follow up (flow-chart).
Prenatal assessment
We chose to review ultrasound data in the first trimester (T1) before the 15th week of amenorrhea (WA), and in the second trimester (T2) between 20 and 28 WA. Ultrasound data collected included the abdominal content of omphalocele, as well as the omphalocele diameter (OD)/ abdominal diameter (AD) ratio. Abdominal content of omphalocele was dispatched in four groups: bowel, liver only, liver and bowel, and liver+ bowel and stomach.
Cardiac ultrasound after 18 WA, prenatal karyotype, Array Comparative Genomic Hybridization (array CGH), and one visit with a pediatric surgeon were offered as part of the routine prenatal management.
Neonatal management
First items of interest were maternal age, mode of delivery, term of delivery (gestational age), and birth weight. Hypotrophy was defined by birth weight ≤10e percentile according to AUDIPOG curve [11].
After birth, all infants underwent complete clinical examination, transfontanellar, abdominal and cardiac ultrasound scanning (US), plain X-Ray of the abdomen and chest, and control of the array CGH. We recorded items of postnatal evaluation (congenital heart disease form, either cyanotic or not, other associated malformations, and genetics results).
Whenever it was possible, herniated viscera were reduced in a 1-stage procedure. Otherwise, progressive techniques were performed. Further items of interest included mortality, discharge from neonatal intensive care unit (NICU), discharge from hospital, surgical technique, length of parenteral and enteral nutrition, length of mechanical ventilation, need for high frequency oscillation (HFO) ventilation or tracheostomy, post-operative complications (pulmonary hypoplasia, pulmonary hypertension, abdominal hypertension), and need for other surgeries such as Nissen fundoplication, gastrostomy and hernia repair.
Analysis
Mortality was recorded as death in the first year of life. Severe morbidity was defined as a composite criterion including at least one of the following grounds: need for enteral feeding by gastrostomy, Nissen fundoplication, HFO ventilation, tracheostomy and/or home oxygen [10]. The data were analyzed using SPSS 23 software. Qualitative variables were compared using Chi-square or Fisher test. Quantitative variables were compared using Student test. Significance was set at p ≤ 0.05.
Population
Two hundred and seventeen consecutives cases were referred in NEM between 2008 and 2018 after prenatal diagnosis of omphalocele before 15 WA. We then excluded 91 termination of pregnancy (42%), 22 in utero fetal demises (10%) and 3 neonatal demises with karyotype abnormality. In 14 cases the diagnosis of omphalocele was inaccurate. Nine were identified prenatally (two gastroschisis, two conjoined twins, and five umbilical cysts) and 5 in the postnatal period (4 vitelline ducts and one hepatic duplication). Twelve patients were lost to follow up. We thus reviewed the medical records of the 75 live newborns actually presenting with omphalocele.
Associated anomalies
Among the 75 infants included, postnatal evaluation identified 46 isolated omphaloceles (61,3%), 12 Wiedemann-Beckwith syndrome (WBS) (16%), 11 associated congenital heart disease (14,8%) and 6 cases (8%) with multiple associated anomalies but no genetic disorder.
Repartition of associated congenital heart disease was ventricular septal defect (VSD) in 6 cases, atrial septal defect (ASD) in 3 cases, VSD associated with ASD in one case and one tetralogy of Fallot.
In polymalformative syndromes, associated anomalies were intra-uterine growth restriction (IUGR) in 4 cases, heart disease in 4 cases (1 ASD, 1 VSD, 1 tetralogy of Fallot and 1 double outlet right ventricle (DORV) associated with VSD), lower extremity deformities in 4 cases, vertebral column anomaly in one case, cerebral septal agenesis in one case.
Obstetrical management
Mean maternal age was 31 +/- 5,7 years old. Sixteen cases (21,3%) were spontaneously vaginally delivered. All these cases had abdominal content with bowel only. The other (78,7%) had either emergency or planned C-section. Overall prematurity was 32% in our study (n=24/75). Prematurity was induced in 11 cases (2 preeclampsia, 3 anomalies in fetal heart monitoring before labor, 3 placental abruption, 2 preterm rupture of fetal membrane, one suspected chorioamnionitis). Spontaneous prematurity occurred in 13 cases. Mean term of birth was 37 WA (+/- 2,74 SA). Mean birth weight was 2841 g (+/- 722 g). Hypotrophy concerned 12 cases.
Surgical management
More than half of the patients were treated with interrupted suture on the muscular then subcutaneous layers, followed by skin closure (n = 40, 53%) between the first and the third day of life. Immediate closure with a non-absorbable patch of synthetic mesh was realized in 8 cases and use of a silon chimney in 26 cases. One case was not operated. We defined giant omphalocele by need of surgical management with patch or synthetic mesh, whatever the extruded viscera observed at birth (two times surgical procedure).
Mortality
Overall mortality was 10,6% (n=8). Median survival length in this group was 28 days (5 days- 6 months). Postnatal evaluation had identified 3 isolated omphalocele, one WBS, 2 associated congenital heart disease and 2 multiple associated anomalies (Table 1).
Table 1. Overall mortality
|
Status |
Abdominal content |
Birth term
(WA) |
Prematurity cause |
Death Cause |
Survival |
Case 1 |
Isolated |
Liver, bowel, stomach |
29 |
Pre-eclampsia |
Oxygene deficiency (bronchopulmonary dysplasia and pulmonary hypoplasia) |
1 week |
Case 2 |
isolated |
Liver, bowel, stomach |
36 |
Fetal heart monitoring abnormalities |
Oxygene deficiency (pulmonary hypoplasia) |
2 months |
Case 3 |
Isolated |
Bowel |
29 |
Spontaneous (Twin) |
Prematurity (bronchopulmonary dysplasia, necrotizing enterocolitis) |
1 month |
Case 4 |
WBS |
Bowel |
27 |
Placental abruption |
Prematurity (bronchopulmonary dysplasia) |
1 month |
Case 5 |
Heart disease (ASD) |
Liver, bowel, stomach |
38 |
- |
Oxygene deficiency (pulmonary hypertension, pulmonary hypoplasia) |
6 months |
Case 6 |
Heart disease (ASD) |
Liver, bowel, stomach |
33 |
Pre-eclampsia |
Oxygene deficiency (Abdominal hypertension, pulmonary hypoplasia) |
3 months |
Case 7 |
Polymalformative (vertebral, IUGR) |
Liver, bowel, stomach |
39 |
- |
Oxygene deficiency (pulmonary hypertension, pulmonary hypoplasia) |
1 month |
Case 8 |
Polymalformative (DORV, ear dysplasia, finger agenesis) |
Liver and bowel |
38 |
- |
Oxygene deficiency (Abdominal hypertension) |
2 weeks |
WA: weeks of amenorrhea; ASD: Atrial septal defect; IUGR: intra uterine growth restriction; DORV: Double outlet right ventricle |
After exclusion of dead patients, neonatal severe morbidity according to our composite criterion occurred in 11/67 cases. Among these 11 patients, postnatal evaluation identified 6 isolated omphaloceles, one WBS, and 4 associated congenital heart disease (Table 2).
Table 2. Overall morbidity
|
Status |
Abdominal content |
Birth term
(WA) |
length of stay in hospital (days) |
length of stay in NICU (days) |
length of mechanical ventilation (days) |
HFO (causes) |
Case 9 |
Isolated |
Liver, bowel, stomach |
36 |
70 |
36 |
27 |
yes (Abdominal hypertension pulmonary hypoplasia) |
Case 10 |
isolated |
Liver and bowel |
38 |
60 |
45 |
28 |
yes (Abdominal hypertension) |
Case 11 |
Isolated |
Liver |
37 |
100 |
30 |
17 |
yes (Abdominal hypertension) |
Case 12 |
Isolated |
Liver |
37 |
69 |
44 |
38 |
yes (Abdominal hypertension) |
Case 13 |
Isolated |
Liver, bowel, stomach |
33 |
65 |
35 |
15 |
Yes (bronchopulmonary dysplasia) |
Case 14 |
Isolated |
Liver, bowel, stomach |
37 |
63 |
50 |
30 |
Yes (pneumopathy) |
Case 15 |
WBS |
bowel |
30 |
60 |
40 |
36 |
yes (bronchopulmonary dysplasia) |
Case 16 |
Heart disease (ASD,VSD) |
Liver, bowel, stomach |
38 |
90 |
78 |
58** |
yes (Abdominal hypertension) |
Case 17 |
Heart disease (VSD) |
Liver and bowel |
39 |
270 |
210 |
100** |
yes (pulmonary hypoplasia) |
Case 18 |
Heart disease (VSD) |
Liver, bowel, stomach |
37 |
210 |
180 |
55** |
yes (pulmonary hypoplasia) |
Case 19 |
Heart disease (Fallot) |
Liver |
29 |
180 |
150 |
75 |
No |
WA: weeks of amenorrhea; ASD: Atrial septal defect; NICU neonatal intensive care unit ; HFO High frequency oscillator; **Nissen and gastrostomy |
Median length of mechanical ventilation in this group was 33 days (15-100 days). HFO ventilation was required for 10/67 infants (15%): 4 cases for hypercapnia that appeared immediately after intra-abdominal hypertension due to surgical procedure, one case for pulmonary hypoplasia, one case for pulmonary infection, two case for broncho-pulmonary dysplasia, and two cases for combined reasons. One infant born at term with giant omphalocele required a tracheostomy.
Median length of parenteral nutrition in this group was 45 days (23-90 days). Three cases needed a gastrostomy and 9 cases still needed an enteral nutrition at home; Nissen fundoplication was performed in 3 cases. Median length of stay hospital and in neonatal intensive care units (NICU) in this group was 20 days (2-270 days) and 6 days (1-210 days), respectively (Figure 1).
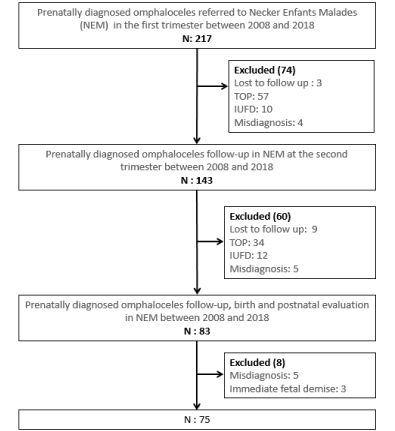
Figure 1. Flow chart
Ultrasound variables
Abdominal content of omphalocele: Before 15 WA, the abdominal content of omphalocele was bowel in 31 cases (41,3%), isolated liver in 18 cases (24%), bowel and liver in 21 cases (28%) and liver, bowel and stomach in 5 cases (6,7%). Between 20 and 28 WA, the abdominal content of omphalocele was bowel in 30 cases (40%), isolated liver in 27 cases (36%), bowel and liver in 8 cases (10,6%) and liver bowel and stomach in 10 cases (13,4%).
All cases with stomach in the first trimester had the same abdominal content in the second trimester. One case with bowel only in the first trimester became with liver only in the second trimester. The abdominal content of thirteen cases with liver and bowel in the first trimester changed in the second trimester, 8 cases became liver only and five cases became liver, bowel and stomach. Repartition of abdominal content according to associated anomalies is reported in Figure 2.
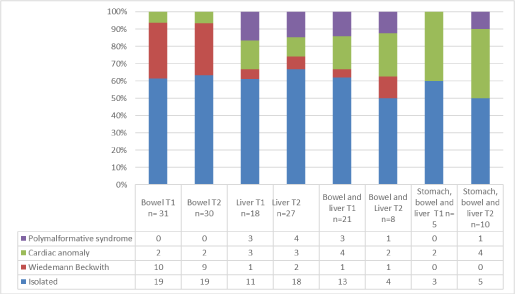
Figure 2. Abdominal content of omphalocele and associated anomalies
Correlation with mortality and morbidity
Content observed <15 WA: For all cases included, the mortality observed was 6% when abdominal content observed in the first trimester was bowel only, 0% when abdominal content was liver only, 23% when abdominal content was bowel and liver and 20% when abdominal content was bowel, liver and stomach.
In the first trimester (<15WA), omphalocele containing liver and bowel was significantly associated with mortality (62,5% (n= 5) vs 23,5% (n= 16) p =0,05) (Table 3).
Table 3. Mortality and morbidity according to ultrasound and neonatal data
|
|
Death
n = 8
N (%) |
Alive
n = 67
N (%) |
p
|
High Morbidity
n = 11
N (%) |
No Morbidity
n = 56
N (%) |
p
|
Ultrasound variables in the first trimester before 15 WA |
Bowel |
2(25) |
29(44) |
0,51 |
1(9,5) |
28(50,2) |
0,03 |
Liver |
0 |
18(26,5) |
0,22 |
1(9,5) |
17(30,3) |
0,27 |
Liver and bowel |
5(62,5) |
16(23,5) |
0,05 |
6(54) |
10(17,8) |
0,03 |
Stomach |
1(12,5) |
4(6) |
0,47 |
3(27) |
1(1,7) |
0,01 |
Ultrasound variables in the second trimester between 20-28 WA |
Bowel |
2(25) |
28(41,8) |
0,59 |
1(9,5) |
27(48,2) |
0,03 |
Liver |
0 |
27(40,2) |
0,06 |
3(27) |
24(42,8) |
0,62 |
Liver and bowel |
1(12,5) |
7(10,5) |
0,85 |
2(18) |
5(9) |
0,64 |
Stomach |
5(62,5) |
5(7,5) |
< 0,001 |
5(45,5) |
0 |
<0,01 |
OD/AD > 0,8 |
4(50) |
8(11) |
0,006 |
5(45,5) |
3(5,3) |
<0,01 |
Neonatal characteristics |
Hypotrophy |
1(12,5) |
11(16,4) |
0,69 |
2(18) |
9(16) |
0,78 |
Prematurity |
5(62,5) |
19(28,3) |
0,09 |
4(36) |
15(26,7) |
0,66 |
Congenital heart disease |
3(37,5) |
12(17,9) |
0,35 |
4(36) |
8(14,3) |
0,16 |
Polymalformative |
2(25) |
4(6) |
0,20 |
0 |
4(7,1) |
0,85 |
Wiedmann Beckwith Sd |
1(12,5) |
14(20,8) |
0,93 |
1(9,5) |
13(23,2) |
0,61 |
AD: abdominal diameter; OD: omphalocele diameter;
WA: weeks of amenorrhea; Sd : syndrome |
After exclusion of dead patients, we included 67 patients to establish correlation with morbidity.
Morbidity affected 3% of the cases when abdominal content was bowel, 5.5% when abdominal content was liver only, 28.5% when abdominal content was bowel and liver and 60% when abdominal content was bowel, liver and stomach.
Abdominal content of omphalocele with liver and bowel observed in the first trimester (<15WA) was significantly associated with morbidity (54% (n=6) vs 17,8% (n=10) p < 0,03) (Table 3).
Content observed between 20 and 28 WA: Overall mortality concerned 6% of the cases when abdominal content was bowel only in the second trimester, none when abdominal content was liver only, 12.5% when abdominal content was bowel and liver and 50% when abdominal content was bowel, liver and stomach.
In the second trimester (20-28 WA), omphalocele containing stomach was significantly associated with mortality (62,5% (n=5) vs 7,5% (n=5) p < 0,001) (Table 3).
Morbidity rate was 3% when abdominal content was bowel only, 11% when abdominal content was liver only, 25% when abdominal content was bowel and liver and 50% when abdominal content was bowel, liver and stomach (Figure 3).
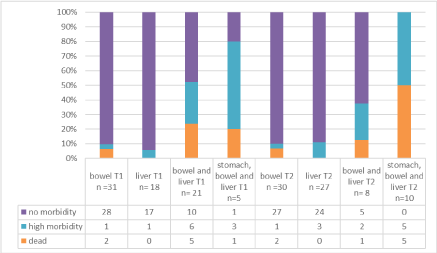
Figure 3. Mortality and morbidity according to abdomen content of omphalocele (all cases)
Abdominal content of omphalocele with stomach in the second trimester (20-28 WA) was significantly associated with morbidity (45,5% (n=5) vs 0%, p < 0,01) (Table 3).
Isolated cases
Content observed <15 WA: For isolated cases, mortality rate was 5.2% when abdominal content was bowel only, 0% when abdominal content was liver only, 15.3% when abdominal content was bowel and liver and 0% when abdominal content was bowel, liver and stomach.
Morbidity rate was 0% when abdominal content was bowel, 0% when abdominal content was liver only, 30.4% when abdominal content was bowel and liver and 66.6% when abdominal content was bowel, liver and stomach.
Content observed between 20 and 28 WA: In isolated cases, morbidity rate was 5.5% when abdominal content was bowel only in the second trimester, 0% when abdominal content was liver only, 0% when abdominal content was bowel and liver and 40% when abdominal content was bowel, liver and stomach.
Morbidity rate was 0% when abdominal content was bowel, 11.1% when abdominal content was liver only, 25% when abdominal content was bowel and liver and 60% when abdominal content was bowel, liver and stomach.
These results are summarized in Figure 4.
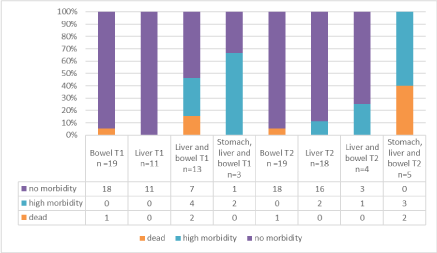
Figure 4. Mortality and morbidity according to abdomen content of omphalocele (isolated cases)
Correlation with OD/AD and associated anomaly: Between 20 and 28 WA (75 patients), 12 cases had an OD/AD ratio > 0,8. OD/AD > 0.8 was significantly associated with mortality (50% (n=4) vs 11 (n=8) p = 0,006) and high morbidity (45.5% (n=5) vs 5.3% (n=3) p<0.01). Congenital heart disease, polymalformative syndrome and Beckwih Wiedemann syndrome was not significantly associated with mortality and high morbidity.
These results are summurized in Table 3.
Correlation with neonatal characteristics: Prematurity and hypotrophy were not significantly associated with overall mortality or high morbidity (Table 3).
This study reports prenatal ultrasound variables associated with morbidity and mortality in a rather large series of patients with omphalocele of all type. There are many antenatal ultrasound variables used to determine the prognosis with high sensitivity (5,6,7,8,9). However, prenatal assessment relying on those may become too pejorative because of a rather low specificity [9]. We thus aimed to find prenatal criteria easier to use for prenatal counseling.
We included all prenatally diagnosed liveborn cases in our institution. According to our laws and parent choices, our center realizes 42% of termination of pregnancy (TOP) when chromosomal anomalies or major congenital associated anomalies are diagnosed. However, many congenital anomalies or genetic syndrome (like WBS) are diagnosed or confirmed only after postnatal screening [12]. Our study population may then be different from other reported series [6,9]. We chose to exclude in utero fetal demise because 11 cases (50%) had a karyotype anomaly, 3 had twin-to-twin transfusion syndrome, 2 had placental dysfunction, 1 had polyhydramnios with premature rupture of membranes and 3 polymalformative syndrome without fetal examination or karyotype. We chose to exclude termination of pregnancy because 50 cases had a karyotype anomaly, 15 cases had a polymalformative syndrome, 15 had a limb body wall complex anomaly, 4 had an atypic Beckwith-Wiedemann syndrome with cerebral anomaly, 2 had an association with complex cardiopathy, one had an association with vertebral column anomaly, one had a Cantrell pentalogy and only three had an isolated omphalocele , out of whom two were associated with twin pregnancy.
The overall survival and morbidity rates (89,4% and 16,4%) are consistent with other series [2]. All patients dead or with high morbidity had multiples factors (prematurity, giant omphalocele, pulmonary hypoplasia, cardiovascular anomalies) associated with greater risk of respiratory failure [13-15]. The presence of liver in the omphalocele was a predictive factor of congenital heart disease, consistent with our previous report [12].
Prematurity rate was high in our series (32%) but did not influence morbidity or mortality in our study. Due to this high prematurity rate, the number of patients with ultrasound evaluation in the third trimester was low. We thus chose not to describe correlation between prenatal ultrasound variables in the third trimester and outcomes.
Only one previous study has described a predicting factor of mortality in literature (OD/AD ≥ 0.26 before 31 WA, OR 4 (95% CI: 1.9-7.5)) [5]. In our study, abdominal content of omphalocele with stomach in the second trimester seems to be a good predictor for neonatal death. Position of stomach was not an effective antenatal variable in the first trimester due to evolution of abdominal content during pregnancy [16] and insufficient number of cases. It is important to pay attention to cases with liver and bowel in the first trimester who may evolve to an enlargement with liver, bowel and stomach in 25% of cases. It is then necessary to distinguish sonographic variables observed in first and second trimester.
About morbidity, in the first trimester an abdominal content of liver and bowel seems to be the best significant predictor for pejorative outcome. Our results are consistent with prior studies demonstrating exteriorized liver as a significant predictor for adverse outcome [6,7], but we clarified this criterion. Indeed, exteriorized liver can range from a small part of liver to the whole organ, so the specificity of liver exteriorization per se is not reliable [6]. Exteriorization of liver and bowel, as a new predicting factor during the first trimester of pregnancy, offers a better specificity. This ultrasound variable is easily reproducible. It allows excluding omphalocele cases with only a small part of liver from the most at-risk patients.
The presence of stomach in second trimester was always associated with poor outcome: 50% of patients died and the other 50% had high morbidity. Pulmonary hypoplasia was associated in 8 cases (80%) and pulmonary hypertension in 6 cases (60%). Causes of death in this group seem to be related to multiple factors (prematurity, associated anomalies, complication of surgical management). Prematurity, vertebral or cardiovascular anomalies (mainly restrictive ASD or VSD) may increase pulmonary hypertension risk and likeliness to have oxygen deficiency.
In the second trimester, exteriorized stomach item was complementary with OD/AD > 0,8 to predict morbidity. This ultrasound variable is more easily reproducible. Correlation between omphalocele content and OD/AD was carried out in supplement 1. In contrast, OD/AD remained the principal method to predict surgical procedure (supplement 2), concurring with Peters and Al study [4].
Abdominal content of omphalocele seems to be useful to guide early prenatal counseling. It is an ultrasound variable easily reproduced but its interpretation may change along pregnancy. The presence of stomach in omphalocele is associated with mortality and high morbidity. This factor is thus of great prognostic value, especially when observed in the first trimester because the pejorative outcome can be early outlined. The presence of liver and bowel in the omphalocele during the first trimester is better than liver only as predicting factor of morbidity. However, abdominal content in these cases is more likely to change along pregnancy, shifting either to more or less pejorative.
None
View Supplementary Data
- Akinkuotu AC, Sheikh F, Olutoye OO, Lee TC, Fernandes CJ, et al. (2015) Giant omphaloceles: surgical management and perinatal outcomes. J Surg Res 198: 388-392. [Crossref]
- Marshall J, Salemi JL, Tanner JP, Ramakrishnan R, Feldkamp ML, et al. (2015) Prevalence, correlates, and outcomes of omphalocele in the United States, 1995-2005. Obstet Gynecol 126: 284-293. [Crossref]
- Kominiarek MA, Zork N, Pierce SM, Zollinger T (2011) Perinatal outcome in the live-born infant with prenatally diagnosed omphalocele. Am J Perinatol 28: 627-634. [Crossref]
- Peters NCJ, Hooft MEV ’t, Ursem NTC, Eggink AJ, Wijnen RMH, et al. (2014) The relation between viscero-abdominal disproportion and type of omphalocele closure. Eur J Obstet Gynecol Reprod Biol 181: 294-299. [Crossref]
- Kiyohara MY, Brizot ML, Liao AW, Francisco RPV, Tannuri ACA, et al. (2014) Should we measure fetal omphalocele diameter for prediction of perinatal outcome? Fetal Diagn Ther 35: 44-50. [Crossref]
- Tassin M, Descriaud C, Elie C, Houfflin Debarge V, Dumez Y, et al. (2013) Omphalocele in the first trimester: prediction of perinatal outcome. Prenat Diagn 33: 497-501.
- Nicholas SS, Stamilio DM, Dicke JM, Gray DL, Macones GA, et al. (2009) Predicting adverse neonatal outcomes in fetuses with abdominal wall defects using prenatal risk factors. Am J Obstet Gynecol 201: 383.e1-6. [Crossref]
- Montero FJ, Simpson LL, Brady PC, Miller RS (2011) Fetal omphalocele ratios predict outcomes in prenatally diagnosed omphalocele. Am J Obstet Gynecol 205: 284.e1-7. [Crossref]
- Peters NCJ, Visser ’t Hooft ME, Eggink AJ, Tibboel D, Ursem N, et al. (2016) Prenatal prediction of the type of omphalocele closure by different medical consultants. Fetal Diagn Ther 39: 40-49. [Crossref]
- Roux N, Jakubowicz D, Salomon L, Grangé G, Giuseppi A, et al. (2018) Early surgical management for giant omphalocele: Results and prognostic factors. J Pediatr Surg 53: 1908-1913. [Crossref]
- Mamelle N, Boniol M, Rivière O (2006) Identification of newborns with Fetal Growth Restriction (FGR) in weight and/or length based on constitutional growth potential. Eur J Pediatr 165: 717-725
- Roux N, Grangé G, Salomon LJ, Rousseau V, Khen-Dunlop N, et al. (2019) Early diagnosis of omphalocele: Prognostic value of the herniated viscera for associated anomalies. Gynecol Obstet Fertil Senol .
- Kamata S, Usui N, Sawai T, Nose K, Fukuzawa M (2008) Prenatal detection of pulmonary hypoplasia in giant omphalocele. Pediatr Surg Int 24: 107-111. [Crossref]
- Partridge EA, Hanna BD, Panitch HB, Rintoul NE, Peranteau WH, et al. (2014) Pulmonary hypertension in giant omphalocele infants. J Pediatr Surg 49: 1767-1770. [Crossref]
- Duggan E, Puligandla PS (2019) Respiratory disorders in patients with omphalocele. Semin Pediatr Surg 28: 115-117.
- Kleinrouweler CE, Kuijper CF, van Zalen-Sprock MM, Mathijssen IB, Bilardo CM, et al. (2011) Characteristics and outcome and the omphalocele circumference/abdominal circumference ratio in prenatally diagnosed fetal omphalocele. Fetal Diagn Ther 30: 60-69. [Crossref]
Editorial Information
Editor-in-Chief
John Livingston Powell
University of North Carolina School of Medicine
USA
Article Type
Research Article
Publication history
Received date: October 10, 2021
Accepted date: October 26, 2021
Published date: October 28, 2021
Copyright
©2021 Roux N. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Roux N, Salomon LJ, Grange G, Beaudoin S (2021) Improving prenatal counseling in omphalocele. Clin Obstet Gynecol Reprod Med 7: DOI: 10.15761/COGRM.1000336




