It is well established in the literature the relationship between structural heart disease and the occurrence of sudden cardiac death. In over 70% of underlying heart disease cases is the ischemic myocardial disease. The pathophysiology stems from the interaction between the event generating electrical instability and the induction of ventricular tachycardia, which degenerates into ventricular fibrillation. The high mortality resulting from the recurrence of these ventricular tachyarrhythmias stimulated the development of several therapies with the objective to prevent the sudden cardiac death, including the surgical approach or radiofrequency ablation aimed at the resection or elimination of the arrhythmogenic focus and the electric treatment by artificial cardiac stimulation with automatic implantable cardiac defibrillator (ICD) and cardiac resynchronization therapy (CRT) [1]. The ICD was introduced in clinical practice in patients who survived cardiac arrest due to ventricular fibrillation or hemodynamically unstable sustained ventricular tachycardia associated with structural cardiopathy, and therefore for secondary prevention of sudden cardiac death [2-6]. Given the first results, Clinical trials aimed at primary prevention, including patients with coronary artery disease and patients with reduced EF, spontaneous non-sustained ventricular tachycardia and sustained ventricular tachycardia induced by the electrophysiological study (EPS) were proposed as eligibility criteria.
In accordance with the latest Brazilian Guidelines for Implantable Electronic Cardiac Devices [7] the ICD reduces mortality and is recommended for primary prevention of sudden cardiac death in patients with ventricular dysfunction in cases of: (i) left ventricular ejection fraction (LVEF) <35% due to myocardial infarction with myocardial infarction for more than 40 days in functional class II or III; (ii) myocardial infarction for more than 40 days, LVEF <30% in functional class I; (iii) Non-sustained ventricular tachycardia due to previous myocardial infarction, LVEF <40%, with ventricular fibrillation or sustained ventricular tachycardia induced by electrophysiological study; and (iv) non-ischemic cardiomyopathy with LVEF <35%, functional class II or III. However, some patients with normal LVEF, areas of myocardial necrosis present episodes of pre-syncope, syncope, monomorphic or polymorphic ventricular ectopic beats in high incidence, non-sustained ventricular tachycardia, needing to be submitted to EPS, to elucidate the symptoms. These areas of necrosis may act as a substrate for the mechanism of reentry of such arrhythmias. The aim of the present study was to compare the incidence of sustained ventricular tachycardia and ventricular fibrillation induction during EPS in patients with cardiac scar zones and low vs. normal LVEF.
This transversal study involved 129 patients with low (n=33) or normal (n=96) LVEF, areas of myocardial necrosis, with a history of episodes of pre-syncope, syncope, monomorphic or polymorphic ventricular ectopic beats in high incidence, non-sustained ventricular tachycardia. The study was piloted in agreement with the Helsinki declaration and approved by the ethics committee of our institution. All patients signed the informed consent term before inclusion.
Study subjects
This study was conducted at the Hospital e Clínica São Gonçalo, Rio de Janeiro, Brazil. Patients were enrolled from January 2015 until April 2017 from the Arrhythmias and Artificial Cardiac Pacing Service of the same hospital. Patients with the combination of the following criteria were consecutively enrolled: (i) a LVEF ≥50% or ≤30% measured by cardiac magnetic resonance image (MRI) using the Simpson’s method, (ii) scar zones detected by cardiac MRI, (iii) episodes of pre-syncope or syncope, (iv) monomorphic or polymorphic ventricular ectopic beats in high incidence, as well as, non-sustained ventricular tachycardia diagnosed on 24-hour-Holter monitoring, (v) age of 18 to 80 years, and (vi) the capacity to read, comprehend, and sign the informed consent form and attend the clinical tests.
The patients that presented any of the subsequent criteria were excluded: (i) pregnancy; (ii) valvular disease with significant adverse sequelae; (iii) unstable angina, myocardial infarction, transient ischemic attack or stroke within the 6 months before the procedure; (iv) a known addiction to drugs or alcohol that affects the intellect; (v) a serious health condition that, in the investigator opinion’s, may adversely affect the safety and/or efficacy of the participant or the study; (vi) treatment with amiodarone; (vii) previous cardiac arrest.
The individuals were divided into two groups (LVEF ≥50%, n=96, and LVEF ≤30%, n=33). All of them underwent to EPS to assess the risk of iducible sustained ventricular tachycardia or ventricular fibrillation. The goal of the present study was to compare the incidence of sustained ventricular tachycardia and ventricular fibrillation induction during EPS in patients with cardiac scar zones and low vs. normal LVEF.
24-hour-Holter monitoring
Patients underwent a 24-hour-Holter monitoring (Galix Biomedical Instrumentation, Florida, USA). A 3-channel recorder was used to record the electrocardiographic traces, calculate the minimum, mean and maximum HR, the quantity and the morphologies of PVCs at baseline [8]. Polymorphic PVCs were defined as 3 or more PVCs of different morphologies in a high incidence each one [8].
Cardiac MRI
Cardiac MRI was performed in all patients at baseline using a 1.5 T Achieva magnetic resonance images (MRI) scanner (Philips Healthcare, Best, the Netherlands) or 1.5 T Siemens Symphony or a 1.5 T Siemens Aera MRI system (Siemens Healthcare Sector, Erlangen, Germany). Cine images were acquired using a balanced steady-state free precession sequence during breath-holds of ~10–15 s using VCG gating with patients being positioned in the supine position. Whole-heart overage from apex to the base was performed as previously reported [9]. Additionally, late gadolinium enhancement (LGE) has been performed in all patients to evaluate the impact of RSD on scar tissue. Late gadolinium-enhanced imaging was performed 10–15 min after injection of 0.2 mmol/kg gadolinium DTPA using an inversion-recovery 3D spoiled gradient echo sequence. The pre-pulse-delay was individually adjusted according to a pre-pulse-delay finder (Look-Locker sequence). All CMR examinations were performed by operators, who were blinded to patient’s treatment.
Cardiac magnetic resonance analysis
Left ventricular mass (LVM) measurements and ejection fraction
Cardiac MRI analyses were performed according to the recommendations of the task force for post-processing of the Society for Cardiovascular MR [10]. Offline Cardiac MRI analyses were performed using the software Qmass MR Enterprise Solution (version 7.4, Medis, the Netherlands). Endocardial and epicardial borders were traced automatically and corrected manually at end-diastole and end-systole, while the papillary muscles were excluded from LVM to achieve better reproducibility [11]. Left ventricular volumes and mass were calculated using the summation of slices method [12]. Left ventricular end-systolic (LVESVI) and end-diastolic volume index (LVEDVI) were normalized in every patient for sex, age, height, and weight, and LVESVI and LVEDVI have been assessed [13]. LVM has then normalized indexing to body surface area (g/m2) [14]. Left ventricular ejection fraction (LVEF) was calculated by Simpson’s method.
Scar tissue
To observe the myocardial fibrosis at baseline, LGE MRI was applied using the inversion-recovery gradient-echo sequence. Late gadolinium-enhanced images were scored visually by two experienced observers (blinded to other MRI and clinical data) at the time of the acquisition using a 17-segment model [15]. Each segment was graded using the following -point score: 0, the absence of enhancement; 1, enhancement of 1–25% transmurality; 2, enhancement of 26–50% transmurality; 3, enhancement of 51–75% transmurality, and 4, enhancement of 76–100% transmurality [16]. The score per segment was then calculated by dividing total score by 17 referring to the 17-segment model [15].
EPS
Under fluoroscopic vision, one right femoral vein puncture was performed, with one 6F sheath. Through the sheath one quadripolar fixed curve catheter was positioned at the tip of the right ventricle; Programmed ventricular stimulation at continuous cycles of 500 and 430 ppm with up to 3 coupled extra-stimuli was performed.
Statistical analysis
The results are expressed as a mean and standard deviation for normally distributed data and as median with interquartile range otherwise. All statistical tests were two-sided. Comparisons between two-paired values were performed with the paired t-test in cases of a Gaussian distribution and by the Wilcoxon test otherwise. Comparisons between more than two-paired values were made by repeated-measures analysis of variance or by Kruskal–Wallis analysis of variance as appropriate, complemented by a post-hoc test. Categorical variables were compared with Fisher’s exact test. A P-value <0.05 was considered significant. Correlations between two variables were performed by Pearson’s chi-square test in case of a Gaussian distribution and with the Spearman correlation test otherwise. All statistical analyses were performed using the program GraphPad Prism v 7.0 (GraphPad Software, La Jolla, CA, USA).
Baseline characteristics of patients
The general features of both groups of patients are listed in Table 1.
Table 1. General features of the patients
Parameters |
LVEF ≤ 30% |
LVEF ≥ 50% |
P value |
N |
33 |
96 |
--- |
Cardiac MRI parameters |
Mean LVEF, % |
24.0 ± 3.7 |
56.5 ± 4.6 |
<0.0001 |
Scar zones, % |
7.3 ± 3.9 |
4.8 ± 2.3 |
<0.0001 |
Uncontrolled hypertension (%) |
13 (40%) |
23 (24%) |
0.1152 |
Age, years |
63.2 ± 10.5 |
58.8 ± 14.3 |
0.1073 |
Body mass index, kg/m2 |
27.2 ± 3.4 |
26.1 ± 5.0 |
0.2432 |
Male gender (%) |
25 (76%) |
76 (79%) |
0.8069 |
White ethnicity (%) |
20 (61%) |
71 (74%) |
0.1845 |
Coronary artery disease (%) |
29 (88%) |
80 (83%) |
0.7808 |
Type 2 Diabetes Mellitus (%) |
9 (27%) |
18 (19%) |
0.3359 |
Antihypertensive |
ACE-inhibitor/ARB (%) |
33 (100%) |
96 (100%) |
>0.9999 |
Diuretic (%) |
25 (76%) |
68 (71%) |
0.6581 |
DHP Ca++ channel blocker (%) |
13 (40%) |
23 (24%) |
0.1152 |
β blocker (%) |
33 (100%) |
96 (100%) |
>0.9999 |
Spironolactone (%) |
33 (100%) |
96 (100%) |
>0.9999 |
Values are presented as mean ± SD or n (%); ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; DHP, dihydropyridine; LVEF, left ventricular ejection fraction; MRI, magnetic resonance image.
EPS - Sustained ventricular tachycardia/ventricular fibrillation induction
During the EPS, all of them (100%) of the 33 patients in the LVEF ≤30% group presented induced sustained ventricular tachycardia/ventricular fibrillation. And, 86 (90%) of the 96 patients in the LVEF ≥50% group developed induced sustained ventricular tachycardia/ventricular fibrillation (P=0.0636) by Fisher’s exact test, presenting no difference between groups regarding the risk of the sudden cardiac death, as shown in Figure 1.
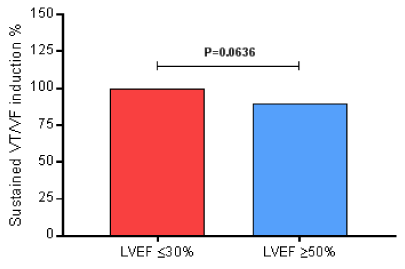
Figure 1. During the EPS, all of them (100%) of the 33 patients in the LVEF ≤30% group presented induced sustained ventricular tachycardia/ventricular fibrillation. And, 86 (90%) of the 96 patients in the LVEF ≥50% group developed induced sustained ventricular tachycardia/ventricular fibrillation (P=0.0636) by Fisher’s exact test; EPS, electrophysiological study; LVEF, left ventricular ejection fraction; VF, ventricular fibrillation; VT, ventricular tachycardia.
Regarding the risk of the sudden cardiac death, there was no difference between groups. We showed that even with normal LVEF patients presenting scar zones in cardiac MRI, clinical symptoms of cardiac low output, and monomorphic or polymorphic ventricular ectopic beats in high incidence, as well as, non-sustained ventricular tachycardia diagnosed on 24-hour-Holter monitoring have the same risk to develop sustained ventricular tachycardia or ventricular fibrillation induction during the EPS, than subjects with low LVEF.
The authors thank all participants of this study and the health insurances from the State of the Rio de Janeiro.
The study was sponsored by health insurances in the state of Rio de Janeiro.
- Nisam S, Mower M, Moser S (1991) ICD clinical update: first decade, initial 10,000 patients. Pacing Clin Electrophysiol 14: 255-262. [Crossref]
- Hallstrom AP, Greene HL, Wyse DG, Zipes D, Epstein AE, et al. (1995) Antiarrhythmics Versus Implantable Defibrillators (AVID)--rationale, design, and methods. Am J Cardiol 75: 470-475. [Crossref]
- Connolly SJ, Gent M, Roberts RS, Dorian P, Green MS, et al. (1993) Canadian Implantable Defibrillator Study (CIDS): study design and organization. CIDS Co-Investigators. Am J Cardiol 72: 103F-108F. [Crossref]
- Kuck KH, Cappato R,2021 Copyright OAT. All rights reservomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest : the Cardiac Arrest Study Hamburg (CASH). Circulation 102: 748-754. [Crossref]
- Mirowski M, Reid PR, Mower MM, Watkins L, Gott VL, et al. (1980) Termination of malignant ventricular arrhythmias with an implanted automatic defibrillator in human beings. N Engl J Med 303: 322-324. [Crossref]
- Maisel WH (2004) Physician management of pacemaker and implantable cardioverter defibrillator advisories. Pacing Clin Electrophysiol 27: 437-442. [Crossref]
- Departamento de Estimulação Cardíaca Artificial da Sociedade Brasileira de Cirurgia Cardiovascular (DECA/SBCCV). Diretrizes Brasileiras de Dispositivos Cardíacos Eletrônicos Implantáveis do Departamento de Estimulação Cardíaca Artificial (DECA) da Sociedade Brasileira de Cirurgia Cardiovascular (SBCCV) 2015. RELAMPA. 2015; 5 de agosto: 1-62.
- Kiuchi MG, E Silva GR, Paz LM, Chen S, Souto GL. (2016) Proof of concept study: renal sympathetic denervation for treatment of polymorphic premature ventricular complexes. J Interv Card Electrophysiol 47: 221-229. [Crossref]
- Kelle S, Roes SD, Klein C, KokocinskiT, de Roos A, et al. (2009) Prognostic value of myocardial infarct size and contractile reserve using magnetic resonance imaging. J Am Coll Cardiol 54:1770–1777. [Crossref]
- Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, et al. (2013) Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson 15:35. [Crossref]
- Vogel-Claussen J, Finn JP, Gomes AS, Hundley GW, Jerosch-Herold M, et al. (2006) Left ventricular papillary muscle mass: relationship to left ventricular mass and volumes by magnetic resonance imaging. J Comput Assist Tomogr 30: 426–432. [Crossref]
- Papavassiliu T, Kuhl HP, van DockumW, Hofman MB, Bondarenko O, et al. (2004) Accuracy of one- and two-dimensional algorithms with optimal image plane position for the estimation of left ventricular mass: a comparative study using magnetic resonance imaging. J Cardiovasc Magn Reson 6: 845–854. [Crossref]
- Maceira AM, Prasad SK, Khan M, Pennell DJ (2006) Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 8: 417–426. [Crossref]
- Armstrong AC, Gidding S, Gjesdal O, Wu C, Bluemke DA, et al. (2012) LV mass assessed by echocardiography and CMR, cardiovascular outcomes, and medical practice. JACC Cardiovasc Imaging 5: 837-848. [Crossref]
- Cerqueira MD,Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, et al. (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Councilon Clinical Cardiology of the American Heart Association. Circulation 105: 539–542. [Crossref]
- Wu E, Judd RM, Vargas JD, Klocke FJ, Bonow RO, et al. (2001) Visualisation of presence, location, and transmural extent of healed Q-wave and non-Q-wave myocardial infarction. Lancet 357: 21-28. [Crossref]

