Many attempts have been made to establish a causal relationship between dentofacial abnormalities and adenotonsillar hypertrophy. We investigated the growth pattern in various planes of the facial profile in children with enlarged adenoids and tonsils by mean of serial cephalometric analyses. Special attention was given to characterize the degree of facial dysmorphology and determine the consequences of surgical intervention. Total 134 children with adenotonsillar hypertrophy who had no history of orthodontic treatment were enrolled. All patients underwent serial cephalometric studies and radiographic landmarks were traced for the angular and linear measurements. The children with adenotonsillar hypertrophy showed a more retrognathic maxilla and mandible against cranial base, a more vertically directed facial growth pattern and higher posterior mandibular inclination than controls. Higher proclination of the upper incisor was observed in children with adenotonsillar hypertrophy aged over 10 years. These differences appeared to be normalized with age in children who underwent adenotonsillectomy at age 4 to 6 years with exception of dental changes. The present study suggests that dentofacial abnormalities are associated with adenotonsillar hypertrophy during development, which shows age-dependent specific morphological alterations.
adenoid, tonsil, craniofacial development, mouth breathing, pediatric, adenoidectomy
The airway, respiratory pattern and craniofacial formation are interrelated and the pharyngeal structures such as adenoid and tonsils play an important role in the adequate facial growth and bony development [1]. The upper airway obstruction during childhood may result in various dentofacial alterations depending on the magnitude, duration and time of occurrence [2]. Therefore, early diagnosis and treatment of any restriction of pharyngeal airway is imperative to normalize form and function and ensure proper orthodontic stability and craniofacial growth.
Hypertrophy of the adenoid and palatine tonsils are one of the most frequent causes of upper respiratory obstruction in pediatric population. Since normal nasal breathing is frequently disrupted in these children, many attempts have been made to establish a causal relationship between dentofacial abnormalities and pharyngeal airway obstruction associated with adenotonsillar hypertrophy [3]. Although investigations have produced mixed results, suggested dentofacial alterations include a constricted maxillary arch, retrognathic mandible, steep mandibular plane angle, increased anterior facial height and malocclusion [4,5].
Potential therapeutic reverse of craniofacial alteration after adenotonsillectomy also has been great concern to the management of children with adenotonsiallr hypertrophy. The change in head and tongue position, accelerated mandibular growth, closure of the mandibular plane angle and improved dental malocclusions have been reported after surgical removal of adenoid and tonsils [6-9]. However, the age factor has not been well characterized when the surgery is considered for the treatment of obstructive adenotonsillar hypertrophy, which may be mandatory to determine surgical timing for the prognosis of craniofacial development. The craniofacial growth has been known to attain 60% of adult size by age 4 and 80~90 % of complete adult size by age 12 [10]. In addition, lymphoid tissue such as adenoid and tonsils, reaches its maximum size between 3 and 6 years of age and then starts gradual involution [11-13]. Therefore, the accelerated craniofacial growth after surgery may not be sufficient to solve the already formed alterations or malocclusion in older children, which may require further orthodontic treatment [14]. A wide spectrum of surgical results across studies could be partly attributed to poor control of age factor because the past studies involve all age groups of children who have different phases of facial growth.
In the present study, we investigated the growth pattern in various planes of the facial profile in children with enlarged adenoids and tonsils by mean of serial cephalometric analyses. Special attention was given to characterize the degree of facial dysmorphology and determine the consequences of surgical intervention.
Study design and participants
Total 134 pediatric patients who were referred for otolaryngological evaluation at CHA University Bundang Hospital between 2008 and 2016 were enrolled and retrospectively reviewed. All patients underwent serial cephalometric studies, which were taken from the age of 4 to 10 years. The children with a history of orthodontic treatment, cranio-facial anomalies and neuro-muscular diseases were excluded. Pediatric patients were divided into three groups: Group I (N=44) with children having pharyngeal airway obstruction with both enlarged adenoids and tonsils, Group II (N=48) with children who underwent adenotonsillectomy between 4-6 years of age and Group III (N=42) with children who had no adenotonsillar hypertrophy as control group. The patients were classified to each group according to the clinical examination and parent-driven questionnaires. The study was approved by the CHA University Bundang Hospital Ethics Committee.
Clinical assessments
Adenoid size was determined based on the A/N ratio of adenoidal depth (A) to nasopharyngeal diameter (N) which was measured on the lateral cephalometric radiograph [12]. Palatal airway patency (PA) was also measured as the shortest distance between the anterior outline of adenoid and the soft palate [15]. In the present study, pathologic adenoidal hypertrophy was defined when there is an A/N ratio greater than 0.8 [12] and PA is measured less than 6 mm [15]. The presence of adenoid hypertrophy was also confirmed using nasopharyngeal endoscopic examination. The tonsils were graded as follows: Grade I, small tonsils confined to the tonsillar pillars; grade II, tonsils that extend just outside the pillars; grade III, tonsils that extend outside the pillars, but do not meet in the midline; grade IV, large tonsils that meet in the midline [16]. The children with tonsillar size more than grade III were considered as having tonsillar hypertrophy.
Cephalometric analysis
Standardized lateral cephalometric radiographs were obtained in all patients. All radiographic images were digitally acquired using a picture archiving and communication system (PACS; Impax: Agfa, Antwerp, Belgium), and assessments were subsequently carried out using PACS software. Dental and skeletal anatomic landmarks used for cephalometric analysis were depicted in figure 1, which were traced for various angular and linear measurements. The anatomic structures were manually demarcated and the cephalometric variables were carefully measured as follows: (1) SNA: angle determined by the intersection of SN and NA lines, (2) SNB: angle determined by the intersection of SN and NB lines, (3) ANB: angle formed by the intersection of NA and NB lines, which measures the relative position of the maxilla to mandible (SNA-SNB), (4) SN-GoGn: angle measuring the inclination of the mandibular plane (GoGn) in relation to the anterior base of the cranium (SN), (5) NSGn (Y axis): angle measuring SN line to gnathion, which is an estimate of mandibular growth direction, (6) U1 to NA angle: the inclination of the long axis of the maxillary central incisor to the N-A line, (7) U1 to NA distance: the distance from the labial surface of the maxillary incisor to the front of N-A line, (8) Interincisal angle (U1-L1): the angle formed between the long axes of the maxillary and mandibular incisors, (9) UAFH: upper anterior face height which is linear measurement from nasion to anterior nasal spine, LAFH: lower anterior face height which is linear measurement from anterior nasal spine to menton.
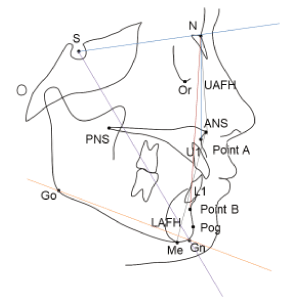
Figure 1. Illustration of anatomic landmarks used for the cephalometric analysis. Sella (S), Nasion (N), Orbitale (Or), Point A: position of deepest concavity on anterior profile of maxilla, Point B: position of deepest concavity on anterior profile of mandible, Pogonion (Pog), Gnathion (Gn), Menton (Me), Anterior Nasal Spine (ANS), Posterior Nasal Spine (PNS), Gonion (Go), Upper Incisor (U1), Lower Incisor (L1), Upper Anterior Facial Height (UAFH): a line connecting Na to ANS, Lower Anterior Facial Height (LAFH): a line connecting ANS to Me.
Statistics
The data were analyzed using IBM SPSS Statistics Version 24 for windows. Measurements are presented as means ± SD. Multiple means were compared among groups as indicators of discriminant validity by ANOVA’s with repeated measures, followed by pair wise post hoc tests. A chi-square test was applied to analyze the significance of the multiple comparison of frequencies among groups. Test results with P < 0.05 were regarded as statistically significant.
Age-related skeletal analysis and postoperative changes
Demographic characteristics of the patients and controls are summarized in table 1. There were no significant differences between patients and controls regarding age or sex ratio.
Table 1. Demographic data of the patients and controls. Group I (n=44), children with enlarged adenoids and tonsils; Group II (n=48), children who underwent adenotonsillectomy between 4-6 years of age and Group III (n=42), children with no adenotonsillar hypertrophy as control group.
Group 1 (n=44) |
Group 2 (n=48) |
Group 3 (n=42) |
P |
Age (months) |
4 yr |
43.3 ± 2.9 |
43.6 ± 3.2 |
44.3 ± 2.8 |
NS |
6 yr |
68.2 ± 3.1 |
67.2 ± 3.0 |
67.4 ± 3.2 |
NS |
8 yr |
83.9 ± 7.2 |
82.9 ± 6.4 |
82.5 ± 6.6 |
NS |
10 yr |
116.3 ± 8.0 |
112.5 ± 8.1 |
113.5 ± 7.4 |
NS |
Female (%) |
16 (36.4) |
20 (41.7) |
16 (38.1) |
NS |
Data are presented as mean ± SD. Significant differences are marked; NS: not significant.
Horizontal cephalometric parameters were measured and plotted against advancing age (Figure 2A-2C). Regarding the measurements of the SNA and SNB, which indicate the antero-posterior position of the maxilla and mandible in relation to the cranial base, the statistical analysis showed that the maxilla and mandible were more retrognathic against cranial base in children with adenotonsillar hypertrophy than control group. The horizontal cephalometric differences decreased with age in children who underwent adenotonsillectomy.
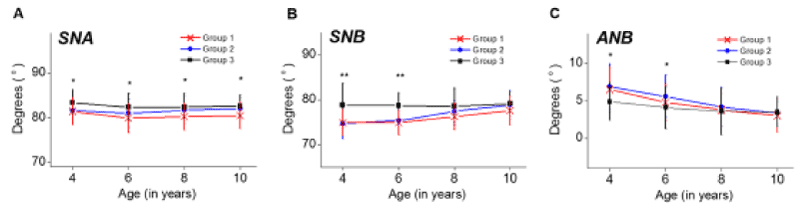
Figure 2. Horizontal cephalometric variables plotted against age. (A) SNA, (B) SNB, (C) ANB. Group I (n=44), children with enlarged adenoids and tonsils; Group II (n=48), children who underwent adenotonsillectomy between 4-6 years of age and Group III (n=42), children with no adenotonsillar hypertrophy as control group. Significant differences are marked, *P<0.05; **P<0.01.
Angular measurements of vertical growth parameters were also plotted against advancing age (Figure 3A and 3B). Children with adenotonsillar hypertrophy presented a higher posterior inclination of the mandible in relation to the cranial base (SN-GoGn) and a more obtuse NSGn angles (Y-axis) across all ages, suggesting an excessive vertical growth facial pattern in children with adenotonsillar hypertrophy. A gradual closure of the mandibular inclination in time has been found in children who underwent adenotonsillectomy.
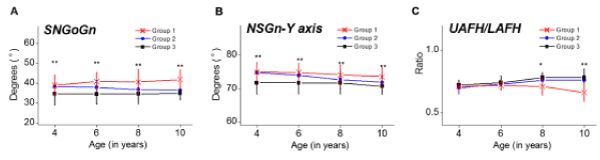
Figure 3. Vertical cephalometric variables plotted against age. (A) SN-GoGn, (B) NSGn, (C) UAFH/LAFH. Group I (n=44), children with enlarged adenoids and tonsils; Group II (n=48), children who underwent adenotonsillectomy between 4-6 years of age and Group III (n=42), children with no adenotonsilar hypertrophy as control group. Significant differences are marked, *P<0.05; **P<0.01.
Linear measurements of facial heights obtained from participants were presented in table 2. The results showed that there was a significant increase in the anterior facial height (N-Me) in children with adenotonsillar hypertrophy aged more than 8 years when compared to control group. Anterior upper and lower facial height (UAFH/LAFH) ratio, which indicates the balance of facial proportions, were statistically lower in children with adenotonsillar hypertrophy aged more than 8 years than control group (Figure 3C).
Table 2. Linear measurements at different chronological age among groups. Group I (n=44), children with enlarged adenoids and tonsils; Group II (n=48), children who underwent adenotonsillectomy between 4-6 years of age and Group III (n=42), children with no adenotonsillar hypertrophy as control group.
| |
Mean score ± SD |
| |
Age |
Group 1 |
Group 2 |
Group 3 |
P |
S-Go (mm) |
4 yr |
65.5 ± 4.7 |
65.3 ± 5.6 |
65.1 ± 5.2 |
NS |
6 yr |
68.5 ± 4.2 |
68.8 ± 6 |
69.6 ± 4.8 |
NS |
8 yr |
71.5 ± 4.1 |
72.7 ± 5.8 |
73.2 ± 5.4 |
NS |
10 yr |
75.4 ± 3.8 |
75.7 ± 4.8 |
76.6 ± 4.9 |
NS |
N-Me (mm) |
4 yr |
104.3 ± 5.9 |
104.6 ± 4.9 |
104.4 ± 5.7 |
NS |
| |
6 yr |
113 ± 4.8 |
112.1 ± 4.8 |
111 ± 6.6 |
NS |
8 yr |
116.9 ± 4 |
115.6 ± 5.4 |
114.5 ± 5.6 |
< 0.05 |
10 yr |
123.1 ± 5.9 |
121.2 ± 6.1 |
120.7 ± 5.9 |
< 0.05 |
Data are presented as mean score ± SD; Significant differences are marked; NS: not significant.
Age-related dental analysis and postoperative changes
Dental parameters were measured and plotted against advancing age (Figure 4A-4C). There was a significant higher proclination of the upper incisor in children with adenotonsillar hypertrophy aged over 10 years than control group. U1NA angles and the measures of U1NA distance were measured significantly higher in older children with adenotonsillar hypertrophy, and the differences were not fully corrected until age of 10 years in children who underwent adenotonsillectomy. Inter-incisal angles were found to be significantly lower in children with adenotonsillar hypertrophy over 10 years of age when compared to controls, indicating proclination of maxillary incisors in these children.
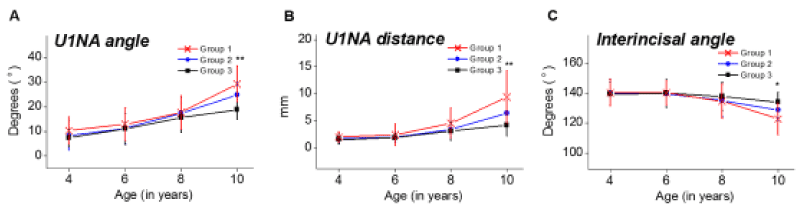
Figure 4. Dental cephalometric variables plotted against age. (A) U1NA angle, (B) U1NA distance, (C) Interincisal angle. Group I (n=44), children with enlarged adenoids and tonsils; Group II (n=48), children who underwent adenotonsillectomy between 4-6 years of age and Group III (n=42), children with no adenotonsillar hypertrophy as control group. Significant differences are marked, *P<0.05; **P<0.01.
The most salient findings of the study are 1) that the children with adenotonsillar hypertrophy have been associated with maxillo-mandibular retrusion in relation to the cranial base, vertically directed facial growth, and higher posterior mandibular inclination, which suggests the influence of the adenotonillar hypertrophy on craniofacial development, 2) that the dental alterations were observed in children with adenotonsillar hypertrophy aged over 10 years, and 3) that the cephalometric differences decreased to a level of controls with age in children who underwent adenotonsillectomy at 4-6 years of age although the surgery seems to have minor effects on dental alterations.
The growth of craniofacial structure is a highly variable complex sequence of epigenetic events, which depends on the various interaction between genetic and environmental factors. There have been studies that link adenoid hypertrophy as a primary etiologic factor to the development of dentofacial abnormalities in children [17-20]. Such dysmorphology can be explained as occurring by changes in head and tongue position and impaired muscular balance. In literatures, it has been reported the acceleration in the growth of the mandible as well as change in its growth direction can be achieved following adenotonsillectomy and overcome skeletal alterations. However, no specific surgical guidelines are yet to be formulated. It would be ideal if adenotonsillectomy would proficiently restore normal functions such as normal breathing, while at the same time preserving the normal craniofacial growth potential. As most formation and/or deformation have already occurred throughout a child's early years of facial growth, appropriate surgical timing of adenotonsillectomy may play an important action on the restoration of the undesirable craniofacial growths. A cross-sectional study had suggested that children with adenoid hypertrophy should be treated before the age of 6 to achieve total normalization of craniofacial growth [21].
In the present study, we carried out temporal analyses of serial cephalometric radiographs during childhood primarily to determine the changes of dentofacial alteration at different growth stages in children with both enlarged adenoid and tonsils. We conducted a study made of groups of single individuals over lengthy periods of time instead of a cross sectional study at a single time point. This method may allow us to properly assess the relative importance of growth periods during specific chronological age and thus suggests an adequate timing for surgical intervention. Our results demonstrated that children with adenotonsillar hypertrophy present skeletal features associated with changes in normal facial proportions, which were comparable with those reported by other authors [9,22-24]. The children with adenotonsillar hypertrophy had a greater posterior inclination of the mandible than controls already at 4 years of age, which indicates a vertical direction of mandibular growth even at an early age. The decreased balance of anterior facial height index in children with adenotonsillar hypertrophy, which was seen over 8 years of age also supports the hypothesis that obstructive adenotonsillar hypertrophy from early childhood could be a etiological factor of induced excessive vertical growth during childhood [25]. After adenotonsillectomy, there was a decrease in the posterior mandibular inclination and an increase in UAFH/LAFH ratio. In literature, the palatal height and overjet were significantly higher while maxillary intermolar width was significantly narrower in children with adenoid hypertrophy [26]. In the present study, there was a significant increase in the upper incisor proclination over 10 years of age in the patient group although it needs further investigation whether this feature is permanent or self-correcting with aging.
Our findings suggest that the skeletal alteration associated with adenotonsillar hypertrophy could be spontaneously corrected during further growth after adenotonsillectomy, if the surgery is provided in young individuals. It is noted that older children with adenotonsillar hypertrophy presented higher tendency towards having more significant changes in facial morphology, occlusal features, and vertically directed facial growth patterns. It seems that if pharyngeal obstruction persists for a long period of time during development, genetically susceptible children would have a more altered craniofacial morphology.
The present study must be interpreted within the limitations. Since the study targeted to the children aged 4 to 10 years with relatively small number of subjects, our study population may not be entirely representative of children with adenotonsillar hypertrophy. Limitations also involved the lack of data from older children who underwent adenotonsillectomy. More knowledge should be accumulated regarding the long-term relationship between adenotonsillar hypertrophy and dentofacial alteration as well as an impact of age on surgical benefits.
In conclusion, the present study demonstrates that dental and skeletal factors are significantly associated with adenotonsillar hypertrophy, which shows dynamic morphological alterations with age. It is important to monitor proper craniofacial growth in children with adenotonsillar hypertrophy and provide early surgical intervention before craniofacial growth spurt.
We would like to express out very great appreciation to Eun A Ahn, for her assistance in the acquisition of data.
- McNamara JA (1981) Influence of respiratory pattern on craniofacial growth. Angle Orthod 51: 269-300. [Crossref]
- O`’Ryan FS, Gallagher DM, LaBanc JP, Epker BN (1982) The relation between nasorespiratory function and dentofacial morphology: A review. Am J Orthod 82: 403-410. [Crossref]
- Warren D, Spalding P (1991) Dentofacial morphology and breathing: a century of controversy. Current Controversies in Orthodontics, Quintessence Pub Co., Chicago, IL: 45-76.
- Warren DW (1990) Effect of airway obstruction upon facial growth. Otolaryngol Clin North Am 23: 699-712. [Crossref]
- Behlfelt K, Linder-Aronson S, Mc William J, Neander P, Laage-Hellman J (1989) Dentition in children with enlarged tonsils compared to control children. Eur J Orthod 11: 416-429. [Crossref]
- Linder-Aronson S, Woodside DG, Lundström A (1986) Mandibular growth direction following adenoidectomy. Am J Orthod 89: 273-284. [Crossref]
- Kerr WJ, McWilliam JS, Linder-Aronson S (1989) Mandibular form and position related to changed mode of breathing--a five-year longitudinal study. Angle Orthod 59: 91-96. [Crossref]
- Weider DJ, Baker GL, Salvatoriello FW (2003) Dental malocclusion and upper airway obstruction, an otolaryngologist's perspective. Int J Pediatr Otorhinolaryngol 67: 323-331. [Crossref]
- Pereira SR, Bakor SF, Weckx LL (2001) Adenotonsillectomy in facial growing patients: spontaneous dental effects. Braz J Otorhinolaryngol 77: 600-604. [Crossref]
- Charles D, (2003) Pediatric otolaryngology. (4th edn), Saunders, Elsevier, USA.
- Songu M, Adibelli ZH, Tuncyurek O, Adibelli H (2010) Age specific size of the upper airway structure in children during development. Ann Otol Rinol Laryngol 119: 541-546. [Crossref]
- Fujioka M, Young LW, Girdany BR (1979) Radiographie evaluation of adenoidal size in children: adenoidal-nasopharyngeal ratio. AJR Am J Roentgenol 133: 401-404. [Crossref]
- Brodsky L, Koch RJ (1993) Bacteriology and immunology of normal and diseased adenoids in children. Arch Otolaryngol Head Neck Surg 119: 821-829. [Crossref]
- Mattar SE, Valera FC, Faria G, Matsumoto MA, Anselmo-Lima WT (2011) Changes in facial morphology after adenotonsillectomy in mouth-breathing children. Int J Paediatr Dent 21: 389-396. [Crossref]
- Maw AR, Jeans WD, Fernando DC (1981) Interobserver variability in the clinical and radiological assessment of adenoid size, and the correlation with adenoid volume. Clin Otolaryngol Allied Sci 6: 317-322. [Crossref]
- Brodsky L, Moore L, Stanievich JF (1987) A comparison of tonsillar size and oropharyngeal dimensions in children with obstructive adenotonsillar hypertrophy. Int J Pediatr Otorhinolaryngol 13: 149-156. [Crossref]
- Flores-Mir C, Korayem M, Heo G, Witmans M, Major MP, et al. (2013) Craniofacial morphological characteristics in children with obstructive sleep apnea syndrome: a systematic review and meta-analysis. J Am Dent Assoc 144: 269-277. [Crossref]
- Katyal V, Pamula Y, Martin AJ, Daynes CN, Kennedy JD, et al. (2013) Craniofacial and upper airway morphology in pediatric sleep-disordered breathing: systematic review and meta-analysis. Am J Orthod Dentofacial Orthop 143: 20-23. [Crossref]
- Joshi MR (1964) A study of dental occlusion in nasal and oronasal breathers in Maharashtrian children. J All India Dent Assoc 36: 219.
- Linder-Aronson S (1979) Respiratory function in relation to facial morphology and the dentition. Br J Orthod 6: 59-71. [Crossref]
- Macari AT, Bitar MA, Ghafari JG (2012) New insights on age-related association between nasopharyngeal airway clearance and facial morphology. Orthod Craniofac Res 15: 188-197. [Crossref]
- Kawashima T, Peltomaki T, Sakata H, Mori K, Happonen RP, et al. (2002) Craniofacial morphology in preschool children with sleep-related breathing disorder and hypertrophy of tonsils. Acta Paediatr 91: 71-77. [Crossref]
- Ung N, Koenig J, Shapiro PA, Shapiro G, Trsk G (1990) A quantitative assessment of respiratory patterns and their effects on dentofacial development. Am J Orthod Dentofac Orthop 98: 523-532. [Crossref]
- Bresolin D, Shapiro GG, Shapiro PA, Dassel SW, Furukawa CT, et al. (1984) Facial characteristics of children who breathe through the mouth. Pediatrics 73: 622-625. [Crossref]
- Lessa FC, Enoki C, Feres MF, Valera FC, Lima WT, et al. (2005) Breathing mode influence in craniofacial development. Braz J Otorhinolaryngol 71: 156-160. [Crossref]
- D'Ascanio L, Lancione C, Pompa G, Rebuffini E, Mansi N, et al. (2010) Craniofacial growth in children with nasal septum deviation: a cephalometric comparative study. Int J Pediatr Otorhinolaryngol 74: 1180-1183. [Crossref]




