Abstract
Despite the improvement in the clinical experience with COVID-19, little is known about COVID-19 in patients with underlying lung disease (ULD) and was it really associated with a poor outcome.
Design
Retrospective comparative cohort study including all patients hospitalized in ICU for COVID-19. Two groups were studied according to the presence or not of ULD. We collected data of demographics, clinical presentation, laboratory results, CT scan findings, treatments and outcomes. The primary outcomes were the use of mechanical ventilation, ICU length of stay and death.
Results
143 patients were included of which 102 were males (ULD group, n=31 versus no ULD group, n=112). ULD corresponded to sleep apnea (SA, n=8), Chronic obstructive pulmonary disease (COPD, n=7), asthma (n=6), diffuse interstitial lung disease (ILD, n=6) and mixed disorder in 4 cases (asthma/SA, n=2 and COPD/SA, n=2).
The ULD group was more likely exposed to tobacco and have more history of bacterial pneumonia. No difference was showed in the clinical presentation. Severity scores and CURB-65 were similar. Only LDH and AST differed, and it were higher in no ULD group. A CT scan involvement greater than 50% was more observed in no ULD group.
The follow up of blood gas, compliance and driving pressure did not reveal any differences between the 2 groups. The 2 groups developed similarly ARDS, thrombo-embolic events and septic shock. Requirement of MV not differed and even duration of MV, the ICU-LOS and the death rate.
Conclusion
Unexpectedly, we didn’t find any major difference (regarding the clinical presentation, severity scores at admission and CURB-65, and the entire disease’s course: ARDS, MV requirement and mortality) between the 2 groups. The differences were showed with LDH and AST levels and CT scan lesions which were most marked with patients without ULD.
Keywords
Covid-19, Acute respiratory distress syndrome,ICU, Asthma, COPD
Abbreviations
ULD: underlying lung disease, SA: sleep apnea , COPD: Chronic obstructive pulmonary disease, ILD: interstitial lung disease, BMI: body mass index ,ARDS: acute respiratory distress syndrome, Covid: Coronavirus disease ,CRP: C-reactive protein, ASAT : Aspartate Aminotransferase , LDH: Lactate dehydrogenase ,CPK: creatine phosphokinase, CT: computed tomography, TAPSE: Tricuspid annular plane systolic excursion, ICU: intensive care unit, MV: mechanical ventilation, PEEP: positive end expiratory pressure, SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2, SOFA: sequential organ failure assessment score. LOS: length of stay
Background
At the end of December 2019, a new coronavirus appeared in Wuhan in China causing a series of cases of viral pneumonia [1]. This new coronavirus is named SARS-CoV-2 for severe acute respiratory syndrome coronavirus-2 by the « Coronaviridae Study Group of the international Committee on taxonomy of viruses » [2].
The disease caused by this virus is named COVID-19 for Coronavirus disease 2019 by the WHO. It became an international health emergency at the end of January 2020 and was qualified as a pandemic in March 2020.
The virus has spread in a few months to the majority of countries in the world causing over 6 millions of confirmed deaths to the date of 1st September 2022 [3].
About 2% of patients with coronavirus disease 2019 (COVID-19) have concomitant pulmonary disease [4]. These patients could be at particular risk for SARS-CoV-2 infection. Thus, exposure to SARS-CoV2 should be reduced given that this category tends to be older with multiple comorbidities, and are often immunosuppressed by their disease or treatment [5].
Infected patients with any underlying chronic lung disease would be expected to be at higher risk of severe complications [6]. However, despite the improvement in the clinical experience with COVID-19, little is known currently about COVID-19 in patients with underlying lung disease (ULD) and was it really associated with a poor outcome.
Studying epidemiological and clinical characteristics of patients with underlying lung disease infected with SARS-CoV-2 is pivotal to clarify determinants of COVID-19 disease severity in ICU patients. In this study, we aimed to evaluate the influence of ULD on both clinical and para-clinical presentation and outcomes of COVID-19.
Patients and methods
Study design and ethical status
it was designed as a retrospective comparative cohort study conducted from September 2020 to February 2021 in the medical intensive care unit (ICU) of the tertiary teaching hospital of la Rabta (Tunis, Tunisia). The local hospital’s Ethics Committee approved the study protocol and informed consent was waived due to the retrospective and non- interventional nature of the study. The anonymity was respected throughout the data processing.
Study population and Data collection
All patients over 18 years old who were admitted in ICU for COVID-19 pneumonia confirmed by RT-PCR or chest imaging were included. No exclusion criteria were considered. For each patient, we extracted age, sex, co morbidities namely the ULD. ULD corresponded to sleep apnea, chronic obstructive pulmonary disease (COPD), asthma, diffuses interstitial lung disease, lung cancer etc. We also recorded the details of clinical presentation, laboratory results, cardiac ultrasound and CT scan findings, follow up of oxygenation and ventilatory behavior (compliance, plateau and driving pressures) from day 3 to day 14, treatments, complications (ARDS, thrombo-embolic events and septic shock) and outcomes.
Study Outcomes
we focused mainly on the use of mechanical ventilation (MV) and the death rate. Secondary outcomes were the ventilatory behavior, complications during the hospitalization, the MV duration and the ICU-length of stay (LOS).
Statistical analysis
We described all baseline and evolutionary characteristics of our retrospective cohort. Next, we divided the population on double cohort according to the presence or not of ULD and compared all the detailed parameters above. Two groups were obtained: critical COVID-19 group with ULD versus critical COVID-19 group without ULD.
Categorical data were provided as frequencies (percentage). Continuous data are provided as means ± standard deviation (SD) or as medians [interquartile range (IQR 25è-75è)] depending on the variable distribution (Gaussian or not).
The different variables studied were compared using the Chi-2 test or the Fisher's exact test for categorical data and Student's t-test or Mann-Whitney test for continuous data.
The statistical significance threshold was set to <0.05. All statistical analyses were performed using IBM SPSS Statistics 20 software.
Results
During the study period, 143 patients were included and among them 31 (22%) had a diagnosis of underlying lung disease and of which 3 required oxygen or ventilation at home. ULD corresponded to sleep apnea (SA, n=8), Chronic obstructive pulmonary disease (COPD, n=7), asthma (n=6), diffuse interstitial lung disease (ILD, n=6) and mixed disorder in 4 cases (asthma/SA, n=2 and COPD/SA, n=2).
Comparison of general Characteristics and Clinical features
Excepting that the ULD group was more likely exposed to tobacco and have more history of bacterial pneumonia than no ULD group, no major difference was showed regarding the demographic criteria neither the clinical presentation: Table 1.
|
All population (n=143) |
ULD group (n=31) |
No ULD group (n=112) |
p |
|
General Characteristics & severity scores |
Age |
63,3+11 |
63+12 |
63+11 |
0,8 |
Sex (M/F) |
102/41 |
22/9 |
80/32 |
1 |
BMI |
28,2+5,8 |
27,8+8 |
28+4 |
0,8 |
Tobacco, n (%) |
31(21%) |
14(45%) |
17(15%) |
0,001 |
History of anterior pneumonia n (%) |
9(6,3%) |
7(22,5%) |
2(3%) |
0,001 |
SAPS II |
32+16 |
28+12 |
33+17 |
0,1 |
SOFA |
5,4+3,8 |
4,9+3,5 |
5,6+3 |
0,38 |
CURB 65 |
1,6+1,1 |
1,65+1 |
1,58+1,1 |
0,78 |
|
Clinical features |
RR |
30+8 |
29+7 |
30+9 |
0,48 |
HR |
91+18 |
86,7+16 |
92+18 |
0,11 |
MAP |
88+17 |
91+14 |
87,6+18 |
0,3 |
Cough n (%) |
92 |
20 |
72 |
1 |
Dyspnea n (%) |
130 |
29 |
101 |
1 |
Chest pain |
7 |
1 |
6 |
1 |
Anosmia |
9 |
1 |
8 |
0,68 |
Arthromyalgia |
64 |
12 |
52 |
0,54 |
Digestive disorders |
26 |
7 |
19 |
0,34 |
Resp struggle signs |
41 |
6 |
35 |
0,26 |
| |
|
|
|
|
|
Table 1. Comparison of Clinical Characteristics; ULD: Underlying Lung Disease, BMI: Body Mass Index, SAPS II: Simplified Acute Physiology Score, SOFA: sequential organ failure assessment score, RR: Respiratory Rate, HR: Heart Rate, MAP: Mean Arterial Pressure
Comparison of para-clinical findings
For laboratory results, only LDH (552 vs 402 IU, p=0,035) and AST (60 vs 38 IU, p=0,016) differed and it were higher in no ULD group. A CT scan involvement greater than 50% was more observed in no ULD group (47% vs 15%, p=0,011): Table 2.
|
All population (n=143) |
ULD group (n=31) |
No ULD group (n=112) |
p |
Laboratory results |
pH |
7,47+0,21 |
7,34 |
7,42 |
0,53 |
pCO2 |
42+18 |
44,5 |
42,2 |
0,5 |
Baseline P/F |
135+98 |
143 |
132 |
0,61 |
HCO3- |
26+5 |
26,6+4 |
26,4+5 |
0,19 |
Lactates |
5,35+3,2 |
3,9 |
5,9 |
0,39 |
WBC |
13088 |
12714 |
13190 |
0,69 |
Lymphocytes |
988 |
833 |
1032 |
0,29 |
CRP |
217 |
166 |
232 |
0,18 |
D dimers |
3608 |
3414 |
3662 |
0,82 |
Fibrinogen |
5,3+1,2 |
4,8 |
6 |
0,67 |
Troponin |
599+167 |
704 |
478 |
0,2 |
CPK |
433 |
249 |
473 |
0,15 |
LDH |
524 |
402 |
552 |
0,035 |
ASAT |
55 |
38 |
59 |
0,016 |
ALAT |
55 |
48 |
57 |
0,44 |
CT scan results |
CT scan involvement > 50%, n (%) |
51 |
4 (15%) |
47(47%) |
0,011 |
LVEF |
65+11 |
61 |
68 |
0,29 |
E/A |
1,3+0,8 |
1,09 |
1,32 |
0,86 |
E/E’ |
10+4 |
11+2 |
9,4+3 |
0,9 |
SPAP |
36+10 |
35,5+11 |
36,5+10 |
0,77 |
TAPSE |
15+4 |
15,7+3 |
15,4+4,2 |
0,86 |
Dilated right cavities |
15 |
4 |
11 |
0,47 |
Table 2. Laboratory and CT scan results; TAPSE: Tricuspid annular plane systolic excursion, LVEF: left ventricular ejection fraction, SPAP: systolic pulmonary artery pressure, CT: computed tomography
Outcomes
Table 3 displayed the different outcomes among the ULD vs no ULD group. The 2 groups developed similarly ARDS, thrombo-embolic events and septic shock. Overall, the mortality was considerable and this independently of the presence or absence of ULD. Requirement of MV and the death rate not differed.
|
All population (n=143) |
ULD group (n=31) |
No ULD group (n=112) |
p |
ARDS: |
Stage 1 |
15 |
3 |
12 |
0,66 |
Stage 2 |
19 |
3 |
16 |
- |
Stage 3 |
80 |
17 |
63 |
- |
MV (%) |
65 |
16 (51%) |
49 (44%) |
0,41 |
Duration of MV (days) |
6,5 |
6,6 |
6,5 |
0,9 |
Vasopressors |
74 |
16 |
58 |
1 |
Pulmonary embolism |
29 |
8 |
21 |
0,63 |
Septic shock |
54 |
14 |
40 |
0,4 |
Acute Kidney injury |
54 |
10 |
44 |
0,53 |
ICU – LOS, (days) |
9,9+6 |
9,1 |
10 |
0,4 |
Death rate (%) |
78 (54,5%) |
16 (51,6%) |
62 (55%) |
0,68 |
Table 3. Outcomes in the study groups; ARDS: acute respiratory distress syndrome, ICU: intensive care unit, MV: mechanical ventilation, LOS: Length of stay
Follow up of oxygenation, compliance and plateau pressure
The follow up of blood gas, compliance and driving pressure did not reveal any differences between the 2 groups: figure 1.
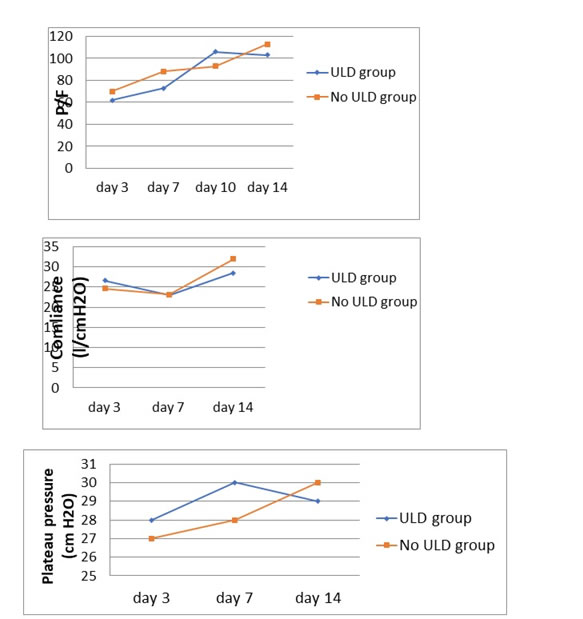
Figure 1. Follow up of oxygenation, compliance and plateau pressure
Discussion
The key findings of our study were that The ULD status did not change the clinical presentation nor worsen the evolutionary process of pneumonia related to COVID-19 in critical patients. All demographic variables and clinical presentation were comparable between the two groups. The proportions of the different outcomes among the ULD vs no ULD group were similar in terms of requirement of Mechanical ventilation, duration of mechanical ventilation and death rate.
There was no association between ULD and ARDS development, thrombo-embolic events and septic shock, nor longer MV duration / ICU stay or increased mortality. Comparatively to patients not having an ULD, those with ULD have less AST and LDH blood levels. A CT scan involvement greater than 50% was in favor of the no ULD group. Overall, the follow up of blood gas, the static respiratory compliance of our population and driving pressure levels did not reveal any differences between the 2 groups.
Prevalence of COVID-19 in patients with chronic respiratory failure
Globally, direct comparisons of the prevalence of ULD among patients with COVID-19 is hard to establish and remain controversial. This can be explained by many bias such as the under-reporting of ULD cases especially at the beginning of the COVID-19 in addition to lack of information and documentation of ULD in the clinical charts [7]. Furthermore, the decrease in lung-functions tests [7] during COVID-19 pandemic led to under-representation of ULD especially COPD and Asthma. In our study, among 143 hospitalized patients at our ICR for COVID-19, 21,7% (31 patients) have ULD among them 8 patients have Sleep Apnea and 7 have COPD. This prevalence is superior to that described in a Nationwide Retrospective Cohort Study of 39,420 Cases in which ULD and ULD overlap was present respectively in 2.8% and 0.2% of patients [8]. We define ULD as the co-existence of two or more individual ULDs. Our prevalence was also higher than the one reported by Beltramo and colleagues (16,03%)
Impact of Chronic Respiratory Diseases on the Outcomes of COVID-19
Findings pertaining to the outcomes of ULD among patients with COVID-19 remain also controversial. In our study, the clinical presentation and the evolutionary process of pneumonia related to COVID-19 were not changed nor worsened by the ULD status in critical patients. However, some authors have reported a negative impact of ULD contrary to our results. A population cohort study conducted by ProfPaul and colleagues [10] found that ULDs (including asthma, COPD, bronchiectasis and idiopathic pulmonary fibrosis) were associated with a higher risk of hospitalization and death. However, the risk of severe COVID-19 in people with asthma is relatively small compared to People with COPD and interstitial lung disease. Similarly, From the Chinese national database, people with COPD followed by Bronchiectasis then Asthma were more likely to have severe or critical illness at hospital admission and reached the composite endpoint within 30 days after hospitalization [10]. COPD is one of the most prevalent ULD followed by Asthma [7] and it is crucial to highlight the association between these two ULD and COVID-19 outcomes and complications. However, COPD, asthma and Pulmonary hypertension were less frequent among COVID-19 patients regardless of age in a nationwide study [8]. A greater susceptibility to contracting SARS-CoV-2 among asthmatic patients (OR, 1.07; 95%CI, 1.00–1.15) and developing adverse clinical outcomes (OR, 1.62; 95%CI, 1.01–2.67) was revealed in a study from Korea that included 220,000 participants compared with those without asthma. [11] As for COPD patients, similarly to smokers, the high expression levels of ACE2 in the small airway epithelium [7] is more likely to expose them to a higher risk of developing severe forms of COVID-19 with more adverse outcomes. Other studies, by contrast to these negative findings, and similarly to our study and our results, no significant difference on SARS-CoV-2 infection, hospital admission, MV requirement or death has been showed between patients with ULD and those without [12]. Another Systematic Review and Meta-Analysis compared outcomes between patients with and without asthma [13] and concluded that patients with Asthma weren’t at higher risk of hospitalization, ICU admission or death and that Asthma was not associated with a higher risk of intubation or mechanical ventilation.
Strength and weakness
Strength
Our study adds data for this pandemic affection in patients with ULD. Indeed, in this population the impact of this pathology remains debated and unresolved. Therefore, multiplying studies in this direction is of extreme interest.
Weakness
We estimate that our study is a retrospective design, small sample and has no individualized analysis for each subtype of ULD.
Conclusions
Our results do not suggest that ULD are associated with more severe COVID-19 presentation. Having ULD was not associated with a greater likelihood of dying from COVID-19 compared with those without ULD. Moreover, neither COPD nor asthma was significantly associated with the risk of death within 30 days after hospitalization [2]. The results of prevalence of COVID-19 among COVID-19 patients remain very distinct worldwide. Patients with ULD were not overrepresented with more intensive treatment, higher clinical severity, or worse outcomes. However, identifying fragile populations with higher susceptibility and worse prognosis is important in the fight against COVID-19
Ethics approval and consent to participate
The study was approved by the local ethics committee of the hospital. The legal representatives of the included patients were informed by the study protocol and gave their consent. The investigation was conforming to the ethical norms of Helsinki declaration.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors Contributions
Salma Ghalloussi and Ahlem Trifi: analysed and interpreted the data, performed the statistical analysis and drafted the manuscript.
Asma Mehdi, Asma Ouhibi and Lynda Messaoud: contributed to the data collection.
Sami Abdellatif: corrected with critical revision of the manuscript
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgement
Not applicable.
References
- Zhou S, Xu J, Sun W, Zhang J, Zhang F, et al. (2021) Clinical Features for Severely and Critically Ill Patients with COVID-19 in Shandong: A Retrospective Cohort Study. Ther Clin Risk Manag 17: 9-21. [Crossref]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses (2020) The Species Severe Acute Respiratory Syndrome-Related Coronavirus: Classifying 2019-NCoV and Naming It SARS-CoV-2. Nat Microbiol 5: 536-544. [Crossref]
- https://www.ccjm.org/content/early/2020/05/12/ccjm.87a.ccc026.
- https://covid19.who.int.
- Antoniou KM, Raghu G, Tzilas V, Bouros D (2020) Management of Patients with Interstitial Lung Disease in the Midst of the COVID-19 Pandemic. Respiration 99: 625-627. [Crossref]
- https://erj.ersjournals.com/content/58/6/2004125.short.
- He Z, Zhong N, Guan W (2022) Impact of Chronic Respiratory Diseases on the Outcomes of COVID-19. Arch Bronconeumol 58: 5-7. [Crossref]
- Aveyard P, Gao M, Lindson N, Hartmann-Boyce J, Watkinson P, et al. (2021) Association between Pre-Existing Respiratory Disease and Its Treatment, and Severe COVID-19: A Population Cohort Study. Lancet Respir Med 9: 909-923. [Crossref]
- Guan W, Liang W, Shi Y, Gan L, Wang H, et al. (2021) Chronic Respiratory Diseases and the Outcomes of COVID-19: A Nationwide Retrospective Cohort Study of 39,420 Cases. J Allergy Clin Immunol Pract 9: 2645-2655. [Crossref]
- Yang JM, Koh HY, Moon SY, Yoo IK, Ha EK, et al. (2020) Allergic Disorders and Susceptibility to and Severity of COVID-19: A Nationwide Cohort Study. J Allergy Clin Immunol Pract 146: 790 98. [Crossref]
- https://www.scopus.com/home.uri.
- Pachón EG, Molina LZ, Sempere MJS, Martínez CB, Delgado JG, et al. (2020) Asma y EPOC en pacientes hospitalizados por COVID-19. Arch Bronconeumol 56: 604-606. [Crossref]
- Liu S, Cao Y, Du T, Zhi Y (2021) Prevalence of Comorbid Asthma and Related Outcomes in COVID-19: A Systematic Review and Meta-Analysis. J Allergy Clin Immunol Pract 9: 693 701. [Crossref]

