Early infantile epileptic encephalopathy 29 (EIEE29) is an autosomal recessive, early onset epileptic encephalopathy in the central nervous system. EIEE29 shows a severe neurologic disease with congenital microcephaly and progressive diffuse cerebral atrophy. Missense mutations of cytoplasmic tRNA catabolic enzyme, alanyl-tRNA synthetase 1 (AARS1), are known to be associated not only with Charcot-Marie-Tooth disease type 2N (CMT2N) but also with EIEE29 [1-3]. Although two types of mutations (Lys81-to-Thr [K81T] and Arg751-to-Gly [R751G]) of AARS1 are responsible for EIEE29 (OMIN No. 616339), it is unknown whether EIEE29-associated AARS1 mutations have pathological effects on neuronal morphological changes and differentiation.
The plasmid encoding the wild type, K81T, or R751G construct (GFP-tagged one) of human AARS1 was transfected into cell lines suitable for transfection (COS-7) [4]. In the Figure S1, the wild type proteins were localized in the cytoplasm. In contrast, the K81T or R751G mutant proteins exhibited punctate structures. To investigate which organelles contain the K81T mutant proteins, we stained cells expressing mutant proteins with an antibody against KDEL antigen (an endoplasmic reticulum antigen; Figure S2), GM130 (a Golgi antigen; Figure S3), or LAMP1 (a lysosome antigen; Figure S4). The K81T mutant proteins resulted in being colocalized with LAMP1 and partially with GM130. Similar results were observed in the R751G mutant proteins (Figures S5-S7).
In addition, it would be of note that neuronal cell line N1E-115 [5] harboring the K81T or R751G mutant constructs exhibited decreased differentiated phenotypes compared with their parental cells (Figures 1 and 2). Further analyses will allow us to promote our understanding not only of the detailed mechanism whereby mutated proteins inhibit differentiation but also of the relationship between EIEE29 mutations and their in vitro pathological effects linking to the in vivo ones.
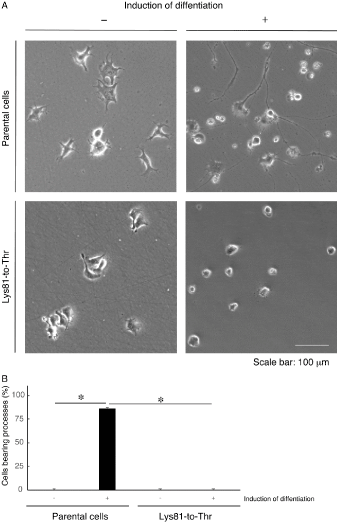
Figure 1. N1E-115 cells harboring the Lys81-to-Thr mutant construct exhibit blunted differentiation. (A) N1E-115 cells stably harboring the AARS1 mutant construct (Lys81-to-Thr) or parental cells were allowed to differentiate for 5 days. (B) Cells with more than one-cell-body length process were considered to be process-harboring, differentiated ones and statistically shown (*, p < 0.01 of one-way ANOVA with post-hoc Fisher’s test; n = 3 fields). Left two bars graphs are data from parental cells and right two ones are data from cells stable clones
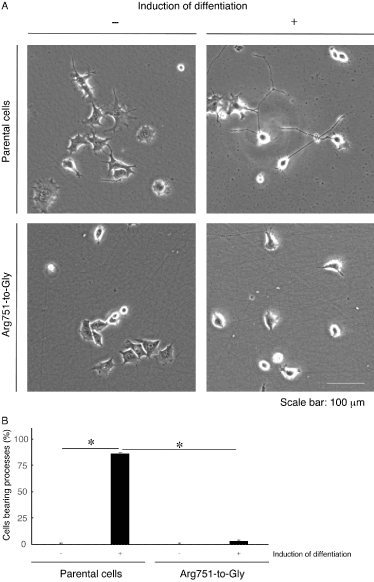
Figure 2. Cells harboring the Arg751-to-Gly mutant construct exhibit blunted differentiation. (A) Cells stably harboring the AARS1 mutant construct (Arg751-to-Gly) or parental cells were allowed to differentiate for 5 days. (B) Cells with more than one-cell-body length process are statistically shown (*, p < 0.01 of one-way ANOVA with post-hoc Fisher’s test; n = 3 fields). Left two bar graphs are data from parental cells and right two ones are data from cells stable clones
- Latour P, Thauvin-Robinet C, Baudelet-Méry C, Soichot P, Cusin V, et al. (2010) A major determinant for binding and aminoacylation of tRNA (Ala) in cytoplasmic alanyl-tRNA synthetase is mutated in dominant axonal Charcot-Marie-Tooth disease. Am J Hum Genet 86: 77-82. [Crossref]
- Lin KP, Soong BW, Yang CC, Huang LW, Chang MH, et al. (2011) The mutational spectrum in a cohort of Charcot-Marie-Tooth disease type 2 among the Han Chinese in Taiwan. PLOS ONE 6: e29393. [Crossref]
- Simons C, Griffin LB, Helman G, Golas G, Pizzino A, et al. (2015) Loss-of-function alanyl-tRNA synthetase mutations cause an autosomal-recessive early-onset epileptic encephalopathy with persistent myelination defect. Am J Hum Genet 96: 675-681. [Crossref]
- Miyamoto Y, Torii T, Tago K, Tanoue A, Takashima S, et al. (2018) BIG1/Arfgef1 and Arf1 regulate the initiation of myelination by Schwann cells in mice. Sci Adv 4: eaar4471.
- Urai YM, Yamawaki N, Watanabe Y, Seki T, Morimoto K, et al. (2018) Yamauchi, Pull down assay for GTP-bound form of Sar1a reveals its activation during morphological differentiation. Biochem Biophys Res Commun 503: 2047-2053.
Editorial Information
Editor-in-Chief
Huasong Zeng
Guangzhou Medical University, China
Article Type
Letter to Editor
Publication history
Received date: August 21, 2020
Accepted date: August 25, 2020
Published date: August 28, 2020
Copyright
©2020 Hattori K. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Hattori K, Takeuchi Y, Tanaka M, Ochiai A, Sawaguchi S (2020) Inhibitory effects of early infantile epileptic encephalopathy 29 (EIEE29)-associated alanyl-tRNA synthetase 1 (AARS1) mutations on neuronal differentiation. J Clin Mol Med 3: doi: 10.15761/JCMM.1000138
Corresponding author
Junji Yamauchi
Laboratory of Molecular Neurology, Tokyo University of Pharmacy and Life Sciences, 1432-1 Horinouchi, Hachioji, Tokyo 192-0392, Japan.
E-mail : bhuvaneswari.bibleraaj@uhsm.nhs.uk

Figure 1. N1E-115 cells harboring the Lys81-to-Thr mutant construct exhibit blunted differentiation. (A) N1E-115 cells stably harboring the AARS1 mutant construct (Lys81-to-Thr) or parental cells were allowed to differentiate for 5 days. (B) Cells with more than one-cell-body length process were considered to be process-harboring, differentiated ones and statistically shown (*, p < 0.01 of one-way ANOVA with post-hoc Fisher’s test; n = 3 fields). Left two bars graphs are data from parental cells and right two ones are data from cells stable clones

Figure 2. Cells harboring the Arg751-to-Gly mutant construct exhibit blunted differentiation. (A) Cells stably harboring the AARS1 mutant construct (Arg751-to-Gly) or parental cells were allowed to differentiate for 5 days. (B) Cells with more than one-cell-body length process are statistically shown (*, p < 0.01 of one-way ANOVA with post-hoc Fisher’s test; n = 3 fields). Left two bar graphs are data from parental cells and right two ones are data from cells stable clones


